Chromate-dichromate system 1. Prepare a clean spot plate and label four wells. Place 5 drops of 0. 10 M K¸CrO̟ into wells 1 and 2, and 5 drops of 0. 10 M K,Cr,0, into wells 3 and 4. 2. Add 2 drops of 2. 0 M H,SO̟ into wells 1 and 3. 3. Add 2 drops of 2. 0 M NaOH into wells 2 and 4. 4. Observe the colors of the solutions. Well 1 2 3 Color of solution Predominant species responsible for color Give the reaction equilibrium that describes this system:
Chromate-dichromate system 1. Prepare a clean spot plate and label four wells. Place 5 drops of 0. 10 M K¸CrO̟ into wells 1 and 2, and 5 drops of 0. 10 M K,Cr,0, into wells 3 and 4. 2. Add 2 drops of 2. 0 M H,SO̟ into wells 1 and 3. 3. Add 2 drops of 2. 0 M NaOH into wells 2 and 4. 4. Observe the colors of the solutions. Well 1 2 3 Color of solution Predominant species responsible for color Give the reaction equilibrium that describes this system:
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.5QAP: Give the advantages and disadvantages of sequential injection analyzers compared to traditional flow...
Related questions
Question
100%
[C] *refer to the (2) photos below* (Please copy the table and answer )
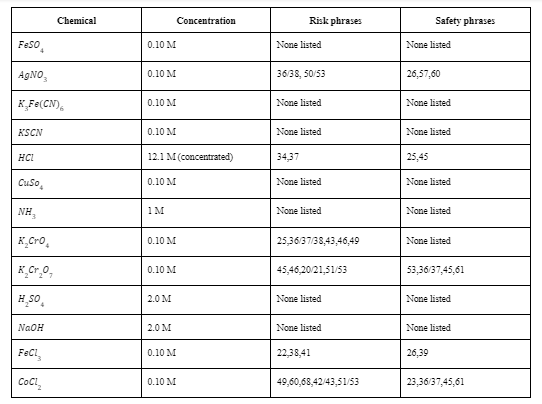
Transcribed Image Text:Chemical
Concentration
Risk phrases
Safety phrases
Feso.
0.10 M
None listed
None listed
AgNO,
0.10 M
36/38, 50/53
26,57,60
KĻF¢(CN),
None listed
0.10 M
None listed
KSCN
0.10 M
None listed
None listed
HCI
12.1 M (concentrated)
34,37
25,45
Cuso,
None listed
0.10 M
None listed
NH,
1м
None listed
None listed
K,Cro,
0.10 M
25,36/37/38,43,46,49
None listed
K_Cr.0,
0.10 M
45,46,20/21,51/53
53,36/37,45,61
H sO,
2.0 M
None listed
None listed
NAOH
2.0 M
None listed
None listed
FeCl,
0.10 M
22,38,41
26,39
CoCl,
0.10 M
49,60,68,42/43,51/53
23,36/37,45,61
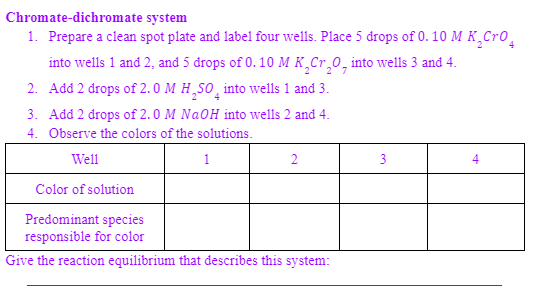
Transcribed Image Text:Chromate-dichromate system
1. Prepare a clean spot plate and label four wells. Place 5 drops of 0. 10 M K,Cro.
into wells 1 and 2, and 5 drops of 0. 10 M K¸Cr,0, into wells 3 and 4.
2. Add 2 drops of 2. 0 M H,SO̟ into wells 1 and 3.
3. Add 2 drops of 2.0 M NaOH into wells 2 and 4.
4. Observe the colors of the solutions.
Well
1
3
4
Color of solution
Predominant species
responsible for color
Give the reaction equilibrium that describes this system:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
