6. Water sample was titrated with EDTA after pH adjustment using Eriochrome black T indicator. Knowing that the concentration of EDTA is 0.050 M and density of water sample equals to 1.10 g/mL. (CaCO,= 100.09 amu).The following results was obtained trials volume of water sample (mL) volume of EDTA (mL) 250.0 250.0 250.0 250.0 10.05 7.00 10.50 10.10 Calculate he total hardness of water as CaCO, and accordingly classify this water. The following results was obtained 1Nm 4
6. Water sample was titrated with EDTA after pH adjustment using Eriochrome black T indicator. Knowing that the concentration of EDTA is 0.050 M and density of water sample equals to 1.10 g/mL. (CaCO,= 100.09 amu).The following results was obtained trials volume of water sample (mL) volume of EDTA (mL) 250.0 250.0 250.0 250.0 10.05 7.00 10.50 10.10 Calculate he total hardness of water as CaCO, and accordingly classify this water. The following results was obtained 1Nm 4
Chapter11: Dynamic Electrochemistry
Section: Chapter Questions
Problem 3P
Related questions
Question
Please answer
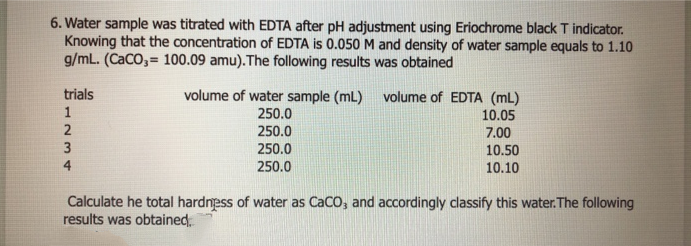
Transcribed Image Text:6. Water sample was titrated with EDTA after pH adjustment using Eriochrome black T indicator.
Knowing that the concentration of EDTA is 0.050 M and density of water sample equals to 1.10
g/mL. (CaCO,= 100.09 amu). The following results was obtained
trials
volume of water sample (mL)
250.0
250.0
250.0
volume of EDTA (mL)
10.05
7.00
10.50
250.0
10.10
Calculate he total hardness of water as CaCO, and accordingly classify this water. The following
results was obtained.
1234
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



