056575718snapshotld=13245488&id-5636490008takeld=4bfba59b09f0e5b& Q Search this course X References Use the References to access important values if needed for this question. Consider the following reaction where K, = 4.55x10 at 723 K: H N2(g) +3H2(g)2NH3(g) 1008 If the three gases are mixed in a rigid container at 723 K so that the partial pressure of each gas is initially one atm, what will happen? A-Z Indicate True (T) or False (F) for each of the following 1. A reaction will occur in which NH (g) is consumed. 2. Kn will increase. 3. A reaction will occur in which N, is consumed 4. Q is less than K. 5. The reaction is at equilibrium. No further reaction will occur 4. Q versus K: This is group attempt 1 of 5 Autosaved at 1:06 PM Back Next 1:06 PM PRA x 11 9/24/2019 Lhp delete 12 prt sc f11 insert KA
056575718snapshotld=13245488&id-5636490008takeld=4bfba59b09f0e5b& Q Search this course X References Use the References to access important values if needed for this question. Consider the following reaction where K, = 4.55x10 at 723 K: H N2(g) +3H2(g)2NH3(g) 1008 If the three gases are mixed in a rigid container at 723 K so that the partial pressure of each gas is initially one atm, what will happen? A-Z Indicate True (T) or False (F) for each of the following 1. A reaction will occur in which NH (g) is consumed. 2. Kn will increase. 3. A reaction will occur in which N, is consumed 4. Q is less than K. 5. The reaction is at equilibrium. No further reaction will occur 4. Q versus K: This is group attempt 1 of 5 Autosaved at 1:06 PM Back Next 1:06 PM PRA x 11 9/24/2019 Lhp delete 12 prt sc f11 insert KA
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 36QAP: At a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s),...
Related questions
Question
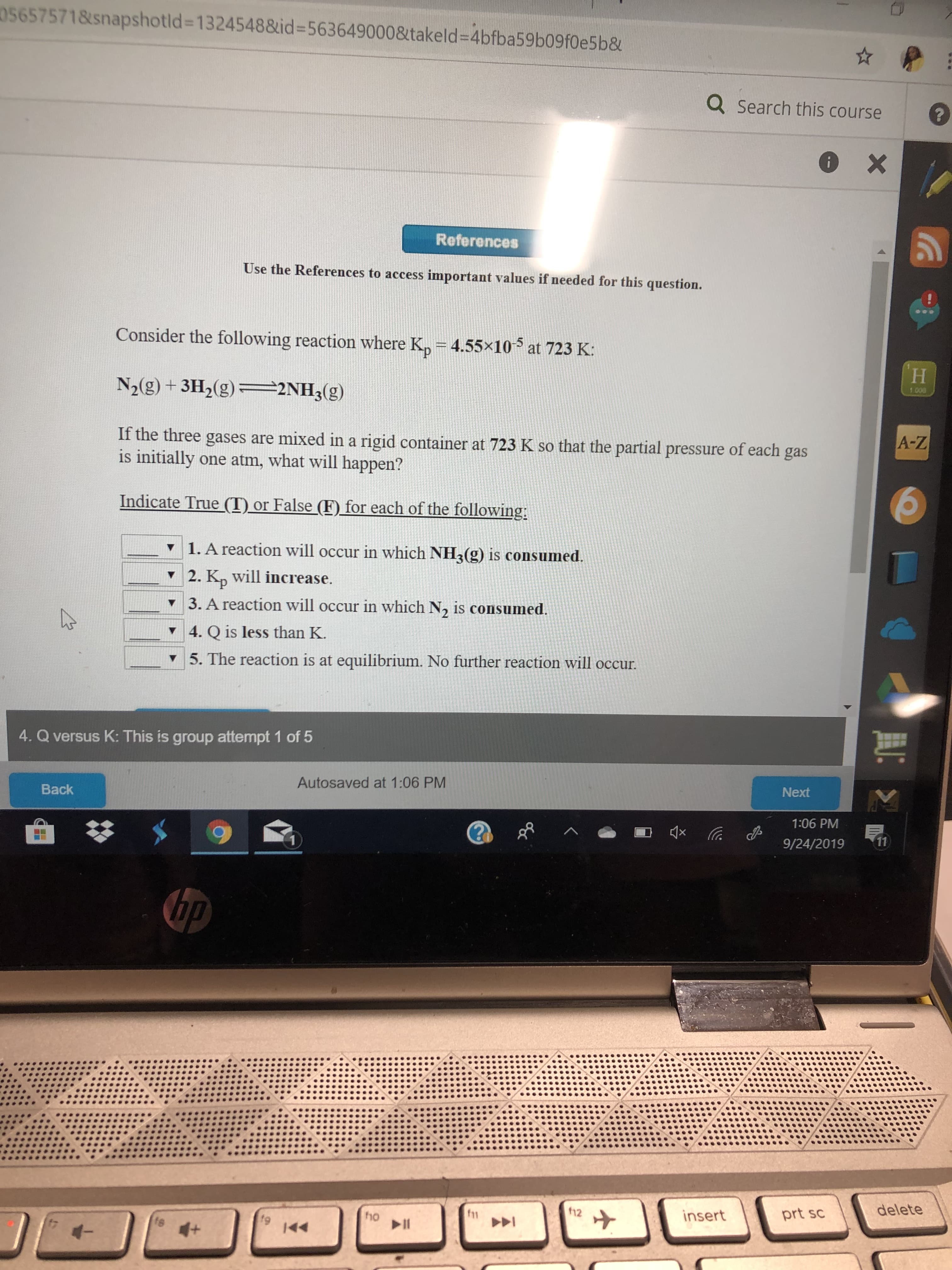
Transcribed Image Text:056575718snapshotld=13245488&id-5636490008takeld=4bfba59b09f0e5b&
Q Search this course
X
References
Use the References to access important values if needed for this question.
Consider the following reaction where K, = 4.55x10
at 723 K:
H
N2(g) +3H2(g)2NH3(g)
1008
If the three gases are mixed in a rigid container at 723 K so that the partial pressure of each gas
is initially one atm, what will happen?
A-Z
Indicate True (T) or False (F) for each of the following
1. A reaction will occur in which NH (g) is consumed.
2. Kn will increase.
3. A reaction will occur in which N, is consumed
4. Q is less than K.
5. The reaction is at equilibrium. No further reaction will occur
4. Q versus K: This is group attempt 1 of 5
Autosaved at 1:06 PM
Back
Next
1:06 PM
PRA
x
11
9/24/2019
Lhp
delete
12
prt sc
f11
insert
KA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning