10. What is the mol fraction of cyclohexane in a mixture containing 15.0 g of toluene and 10.0 g cyclohexane. (Molecular mass of toluene = 92.14 g/mol and that of cyclohexane = 84.16 g/mol) 1). 0.12 2). 0.42 3). 0.58 4). 0.28 11. It is important to wear eye protection in the laboratory because: 1). it looks cool 2. to avoid eye injury 3). to meet the NY State mandate 4). to make it easy to follow the experiment
10. What is the mol fraction of cyclohexane in a mixture containing 15.0 g of toluene and 10.0 g cyclohexane. (Molecular mass of toluene = 92.14 g/mol and that of cyclohexane = 84.16 g/mol) 1). 0.12 2). 0.42 3). 0.58 4). 0.28 11. It is important to wear eye protection in the laboratory because: 1). it looks cool 2. to avoid eye injury 3). to meet the NY State mandate 4). to make it easy to follow the experiment
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 93AP
Related questions
Question
Can you please answer these three sub problems
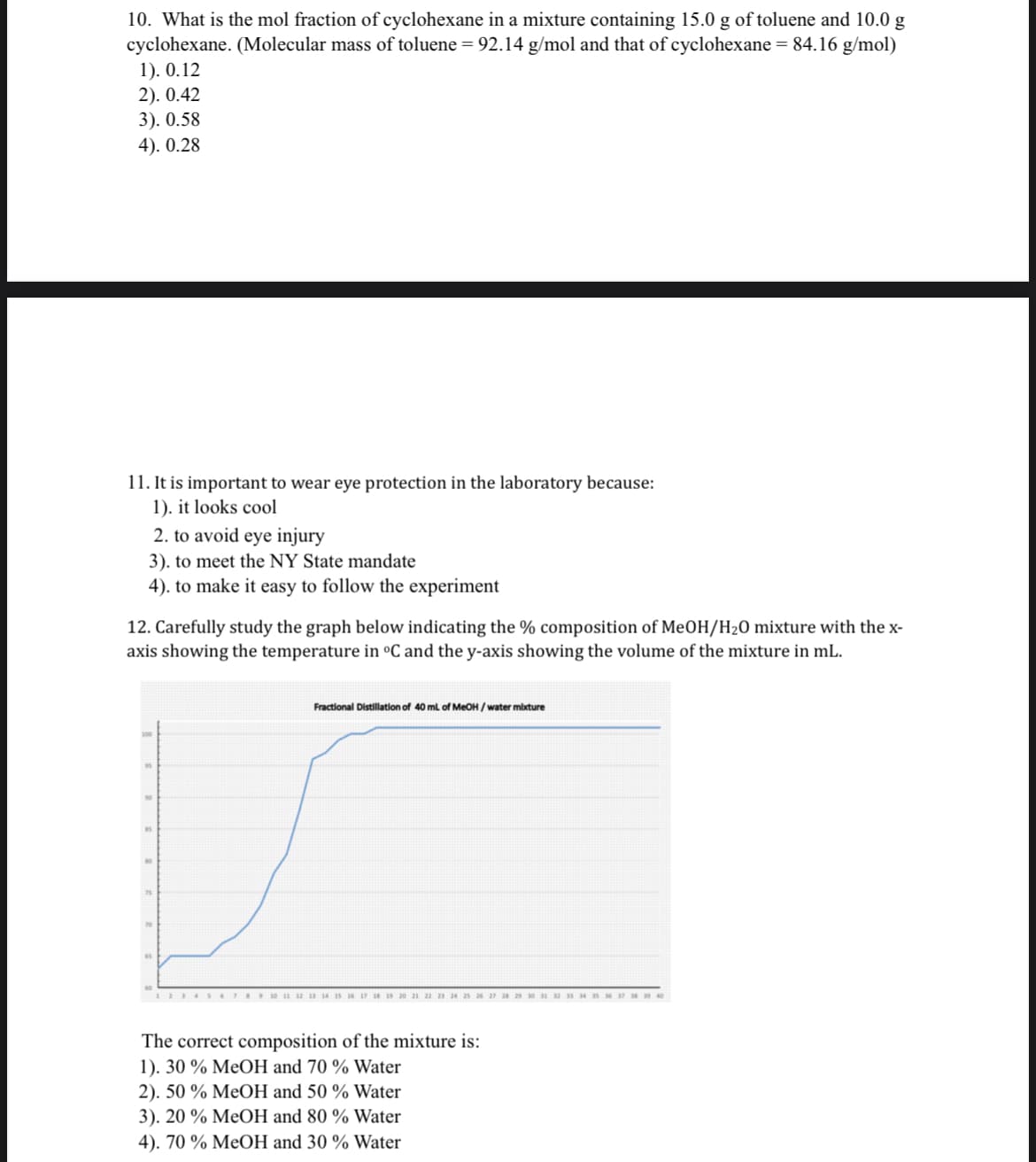
Transcribed Image Text:10. What is the mol fraction of cyclohexane in a mixture containing 15.0 g of toluene and 10.0 g
cyclohexane. (Molecular mass of toluene = 92.14 g/mol and that of cyclohexane = 84.16 g/mol)
1). 0.12
2). 0.42
3). 0.58
4). 0.28
11. It is important to wear eye protection in the laboratory because:
1). it looks cool
2. to avoid eye injury
3). to meet the NY State mandate
4). to make it easy to follow the experiment
12. Carefully study the graph below indicating the % composition of MeOH/H20 mixture with the x-
axis showing the temperature in °C and the y-axis showing the volume of the mixture in mL.
Fractional Distillation of 40 ml of MEOH / water mbture
12345 STESI0 2 is 1 17 19 20 21 22 23 a 25 2 27 2 29 SM
The correct composition of the mixture is:
1). 30 % MeOH and 70 % Water
2). 50 % MeOH and 50 % Water
3). 20 % MeOH and 80 % Water
4). 70 % MeOH and 30 % Water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT