2. A system containing 3.0 moles of a perfect gas with C.,m=2.5 R is put through the following сycle: Step 1, point A to point B: The gas starts at 4.0 atm and 20.0 L and undergoes isothermal expansion to 50.0 L. Step 2, point B to point C: The gas undergoes adiabatic compression to 30.0 L Step 3, point C to point D: The gas undergoes an isochoric process changing the pressure to 4.0 atm. Step 4, point D to point A: The gas undergoes an isobaric process changing the volume to 20.0 L. a) Sketch the cycle on a P-V diagram and label each step. b) Determine the pressure, volume, and temperature at points A, B, C, and D of this cycle. c) Calculate the work for step 1. d) For each step in the cycle, and for each of the quantities q, w, AU, and AH, state whether the value is positive, negative, or zero.
2. A system containing 3.0 moles of a perfect gas with C.,m=2.5 R is put through the following сycle: Step 1, point A to point B: The gas starts at 4.0 atm and 20.0 L and undergoes isothermal expansion to 50.0 L. Step 2, point B to point C: The gas undergoes adiabatic compression to 30.0 L Step 3, point C to point D: The gas undergoes an isochoric process changing the pressure to 4.0 atm. Step 4, point D to point A: The gas undergoes an isobaric process changing the volume to 20.0 L. a) Sketch the cycle on a P-V diagram and label each step. b) Determine the pressure, volume, and temperature at points A, B, C, and D of this cycle. c) Calculate the work for step 1. d) For each step in the cycle, and for each of the quantities q, w, AU, and AH, state whether the value is positive, negative, or zero.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 81AP
Related questions
Question
100%
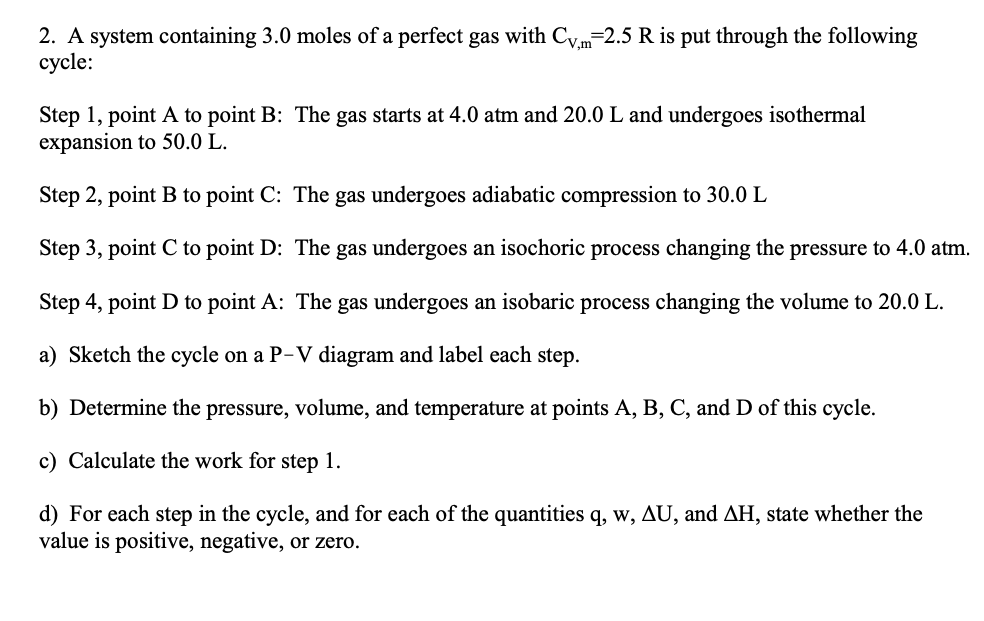
Transcribed Image Text:2. A system containing 3.0 moles of a perfect gas with C.,m=2.5 R is put through the following
сycle:
Step 1, point A to point B: The gas starts at 4.0 atm and 20.0 L and undergoes isothermal
expansion to 50.0 L.
Step 2, point B to point C: The gas undergoes adiabatic compression to 30.0 L
Step 3, point C to point D: The gas undergoes an isochoric process changing the pressure to 4.0 atm.
Step 4, point D to point A: The gas undergoes an isobaric process changing the volume to 20.0 L.
a) Sketch the cycle on a P-V diagram and label each step.
b) Determine the pressure, volume, and temperature at points A, B, C, and D of this cycle.
c) Calculate the work for step 1.
d) For each step in the cycle, and for each of the quantities q, w, AU, and AH, state whether the
value is positive, negative, or zero.
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning