3. Consider the following reactions: ATP + H20 --> ADP + Pi AGO' = -30.5 kJ/mol 1,3-biphosphoglycerate + H20 ----> phosphoglycerate + Pj AGO' = -49.4 kJ/mol a. How many moles of ATP could be produced under standard conditions from the oxidation of one mole of 1,3-biphosphoglycerate assuming 27.3% efficiency? b. Calculate the AGO for the reaction: phosphoglycerate + ATP ----> 1,3-biphosphoglycerate + ADP
3. Consider the following reactions: ATP + H20 --> ADP + Pi AGO' = -30.5 kJ/mol 1,3-biphosphoglycerate + H20 ----> phosphoglycerate + Pj AGO' = -49.4 kJ/mol a. How many moles of ATP could be produced under standard conditions from the oxidation of one mole of 1,3-biphosphoglycerate assuming 27.3% efficiency? b. Calculate the AGO for the reaction: phosphoglycerate + ATP ----> 1,3-biphosphoglycerate + ADP
Biology: The Dynamic Science (MindTap Course List)
4th Edition
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Chapter7: Cellular Respiration: Harvesting Chemical Energy
Section: Chapter Questions
Problem 2TYK
Related questions
Question
help
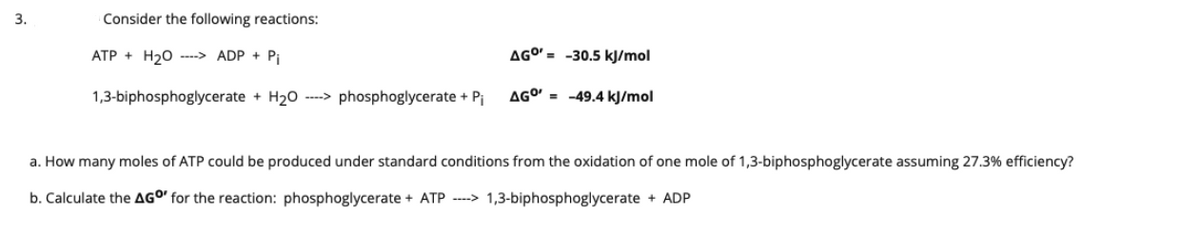
Transcribed Image Text:3.
Consider the following reactions:
ATP + H20 ----> ADP + Pi
AGO' = -30.5 kJ/mol
1,3-biphosphoglycerate + H20 --> phosphoglycerate + Pi
AGO' = -49.4 kJ/mol
a. How many moles of ATP could be produced under standard conditions from the oxidation of one mole of 1,3-biphosphoglycerate assuming 27.3% efficiency?
b. Calculate the AGO' for the reaction: phosphoglycerate + ATP --> 1,3-biphosphoglycerate + ADP
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College