4 g 5 g 28 100 g R Lactic acid Salicylic acid Trichloroacetic acid Flexible collodion qs ad Sig: wart remover. Use as directed. (a) Flexible collodion contains 20% w/w camphor and 30% w/w castor oil. How many grams of each would be contained in 30 g of the mixture? (b) The specific gravity of castor oil is 0.955. How many milliliters of the oil is contained in 30 g of the mixture? (c) If the specific gravity of the mixture is 0.781, what are the percent w/v con- centrations of lactic acid, salicylic acid, and trichloroacetic acid in the mixture?
4 g 5 g 28 100 g R Lactic acid Salicylic acid Trichloroacetic acid Flexible collodion qs ad Sig: wart remover. Use as directed. (a) Flexible collodion contains 20% w/w camphor and 30% w/w castor oil. How many grams of each would be contained in 30 g of the mixture? (b) The specific gravity of castor oil is 0.955. How many milliliters of the oil is contained in 30 g of the mixture? (c) If the specific gravity of the mixture is 0.781, what are the percent w/v con- centrations of lactic acid, salicylic acid, and trichloroacetic acid in the mixture?
Chapter28: Atomic Spectroscopy
Section: Chapter Questions
Problem 28.13QAP
Related questions
Question
answers are:
(a) 5.34 g camphor and 8.01 g castor oil
(b) 8.39 mL castor oil
(c) 3.12% w/v lactic acid,
3.91% w/v salicylic acid, and 1.56% w/v trichloroacetic acid
i want to know the full solutions. thank you!
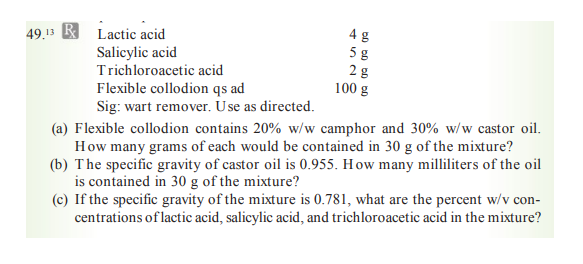
Transcribed Image Text:49.13 B Lactic acid
5 g
28
100 g
Salicylic acid
Trichloroacetic acid
Flexible collodion qs ad
Sig: wart remover. Use as directed.
(a) Flexible collodion contains 20% w/w camphor and 30% w/w castor oil.
How many grams of each would be contained in 30 g of the mixture?
(b) The specific gravity of castor oil is 0.955. How many milliliters of the oil
is contained in 30 g of the mixture?
(c) If the specific gravity of the mixture is 0.781, what are the percent w/v con-
centrations of lactic acid, salicylic acid, and trichloroacetic acid in the mixture?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning