5.Benzoic acid is not very soluble in water and has a much stronger affinity for organic solvents. Considering the reaction below, which of the following best describes what will happen when a base is added to a liquid-liquid extraction system containing water, benzoic acid, and an organic solvent like hexane? Please note that states of matter have been omitted from the reaction. Assume excess NaOH is added.
5.Benzoic acid is not very soluble in water and has a much stronger affinity for organic solvents. Considering the reaction below, which of the following best describes what will happen when a base is added to a liquid-liquid extraction system containing water, benzoic acid, and an organic solvent like hexane? Please note that states of matter have been omitted from the reaction. Assume excess NaOH is added.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter18: Carboxylic Acids
Section: Chapter Questions
Problem 18.28P: 18-28 Arrange these compounds in order of increasing acidity: benzoic acid, benzyl alcohol, phenol.
Related questions
Question
5.Benzoic acid is not very soluble in water and has a much stronger affinity for organic solvents. Considering the reaction below, which of the following best describes what will happen when a base is added to a liquid-liquid extraction system containing water, benzoic acid, and an organic solvent like hexane? Please note that
A.Sodium benzoate will be in the aqueous layer.
B.Sodium hydroxide will be in the organic layer.
C.Benzoic acid will be in the aqueous layer.
D.Water will be in the organic layer.
E.Sodium benzoate will be in the organic layer.
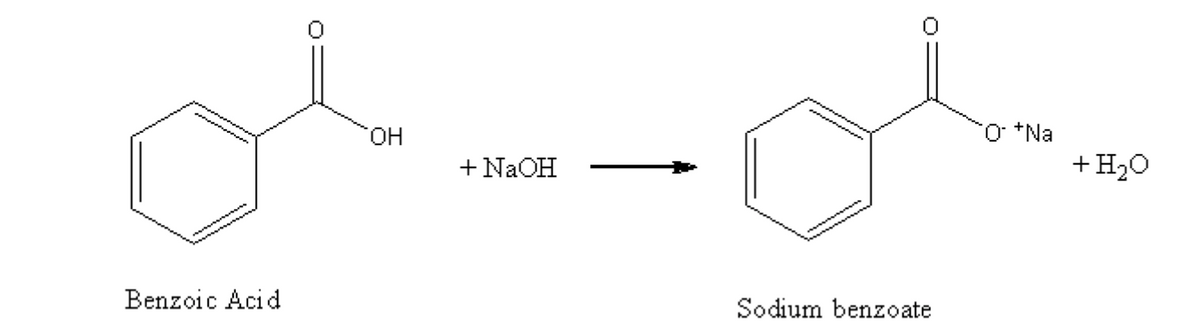
Transcribed Image Text:HO.
+ NAOH
+H20
Benzoic Acid
Sodium benzoate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
