(b) Describe the change in composition of the solution containing a mole fraction of 0.10 A as the temperature increases from 132 to 133°C? 138 136 134 132 130 0.00 0.20 0.40 0.60 0.80 1.00 XA . Why must the spot be applied to the TLC plate above the level of development solvent? A Grignard reaction of phenylmagnesium bromide with 3-pentanone gives 3-phenylpentan- 3-ol as the major product (we will discuss this reaction later). The crude product contains the 3-phenylpentan-3-ol product, unreacted 3-pentanone, and biphenyl (a side product). A developing solvent is found that separates the mixture into three spots on a silica gel TLC plate. Considering the functional groups present, predict which compounds would have the smallest and largest Rf values. Briefly justify your answer. Temperature (°C)
(b) Describe the change in composition of the solution containing a mole fraction of 0.10 A as the temperature increases from 132 to 133°C? 138 136 134 132 130 0.00 0.20 0.40 0.60 0.80 1.00 XA . Why must the spot be applied to the TLC plate above the level of development solvent? A Grignard reaction of phenylmagnesium bromide with 3-pentanone gives 3-phenylpentan- 3-ol as the major product (we will discuss this reaction later). The crude product contains the 3-phenylpentan-3-ol product, unreacted 3-pentanone, and biphenyl (a side product). A developing solvent is found that separates the mixture into three spots on a silica gel TLC plate. Considering the functional groups present, predict which compounds would have the smallest and largest Rf values. Briefly justify your answer. Temperature (°C)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section13.5: Colloids
Problem 1.1ACP: The blue line on the diagram illustrates the effect of using fractional distillation to separate a...
Related questions
Question
can you help with number 4
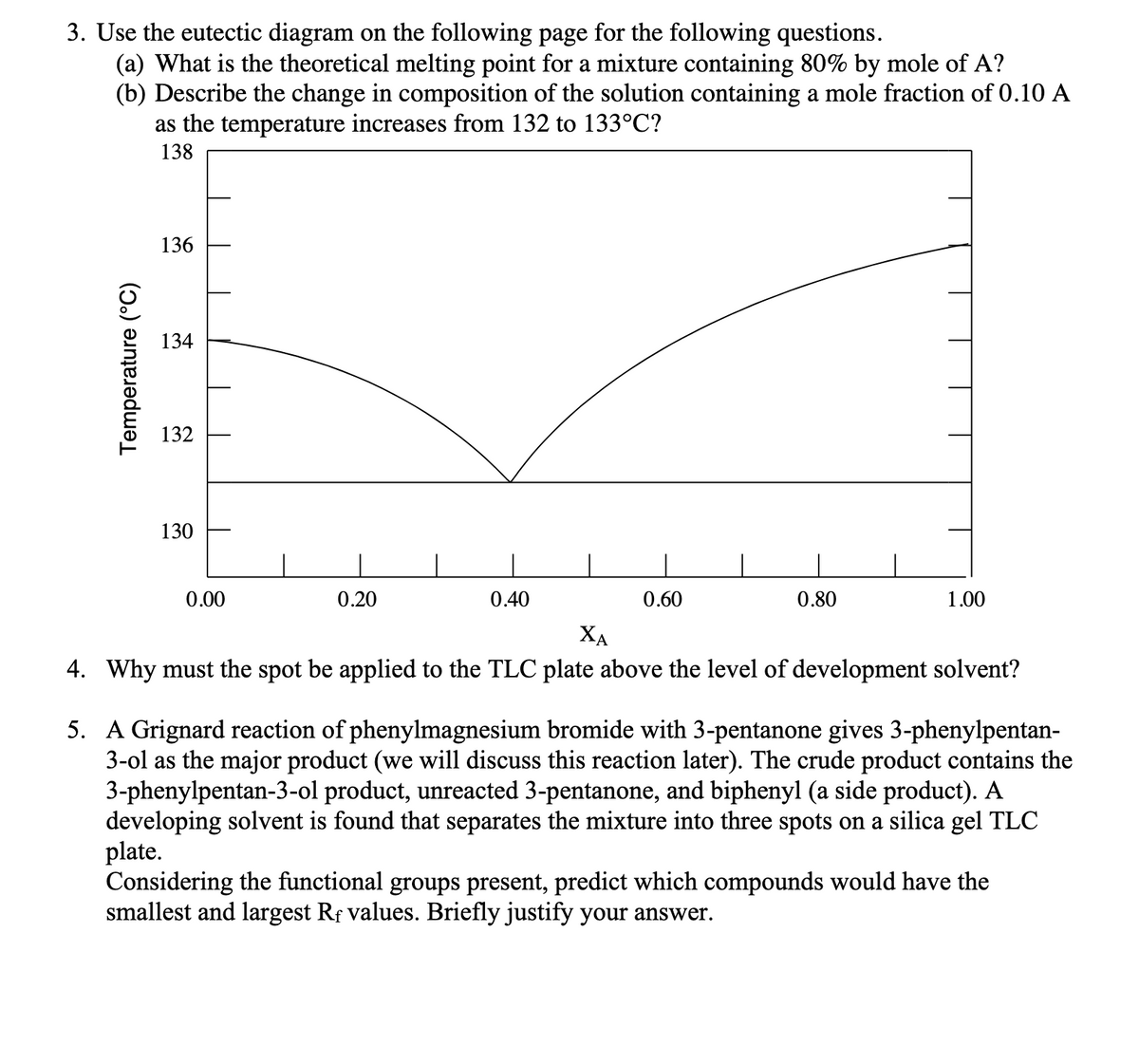
Transcribed Image Text:3. Use the eutectic diagram on the following page for the following questions.
(a) What is the theoretical melting point for a mixture containing 80% by mole of A?
(b) Describe the change in composition of the solution containing a mole fraction of 0.10 A
as the temperature increases from 132 to 133°C?
138
136
134
132
130
0.00
0.20
0.40
0.60
0.80
1.00
XA
4. Why must the spot be applied to the TLC plate above the level of development solvent?
5. A Grignard reaction of phenylmagnesium bromide with 3-pentanone gives 3-phenylpentan-
3-ol as the major product (we will discuss this reaction later). The crude product contains the
3-phenylpentan-3-ol product, unreacted 3-pentanone, and biphenyl (a side product). A
developing solvent is found that separates the mixture into three spots on a silica gel TLC
plate.
Considering the functional groups present, predict which compounds would have the
smallest and largest Rf values. Briefly justify your answer.
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,