A student carries out the equilibrium constant experiment following a similar procedure used in the LearnSmart Lab simulator. In the first part of the experiment, there is a contaminant which causes absorbance values to read higher than they should for the thiocyanatoiron(III) complex. The resulting Beer's law plot has a slope that is larger than it would be if the contaminant was not present. This results in a Beer's Law plot with a slope that is larger than it would be if the contaminant were not present. If the same contaminant was not present when the student carries out the second part of the experiment, how will the student's results be affected? The molarities of thiocyanatoiron(III) ion would calculate higher and the calculated K̟ values would calculate higher. The molarities of thiocyanatoiron(III) ion would calculate lower and the calculated K, values would calculate lower. The molarities of thiocyanatoiron(III) ion would calculate higher and the calculated K̟ values would calculate lower. The molarities of thiocyanatoiron(III) ion would calculate lower and the calculated K, values would calculate higher.
A student carries out the equilibrium constant experiment following a similar procedure used in the LearnSmart Lab simulator. In the first part of the experiment, there is a contaminant which causes absorbance values to read higher than they should for the thiocyanatoiron(III) complex. The resulting Beer's law plot has a slope that is larger than it would be if the contaminant was not present. This results in a Beer's Law plot with a slope that is larger than it would be if the contaminant were not present. If the same contaminant was not present when the student carries out the second part of the experiment, how will the student's results be affected? The molarities of thiocyanatoiron(III) ion would calculate higher and the calculated K̟ values would calculate higher. The molarities of thiocyanatoiron(III) ion would calculate lower and the calculated K, values would calculate lower. The molarities of thiocyanatoiron(III) ion would calculate higher and the calculated K̟ values would calculate lower. The molarities of thiocyanatoiron(III) ion would calculate lower and the calculated K, values would calculate higher.
Chapter12: Spectrochemical Methods
Section: Chapter Questions
Problem 16P
Related questions
Question
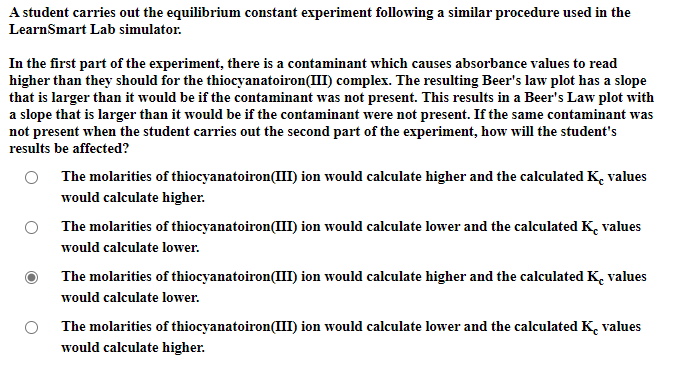
Transcribed Image Text:A student carries out the equilibrium constant experiment following a similar procedure used in the
LearnSmart Lab simulator.
In the first part of the experiment, there is a contaminant which causes absorbance values to read
higher than they should for the thiocyanatoiron(III) complex. The resulting Beer's law plot has a slope
that is larger than it would be if the contaminant was not present. This results in a Beer's Law plot with
a slope that is larger than it would be if the contaminant were not present. If the same contaminant was
not present when the student carries out the second part of the experiment, how will the student's
results be affected?
The molarities of thiocyanatoiron(III) ion would calculate higher and the calculated K. values
would calculate higher.
The molarities of thiocyanatoiron(III) ion would calculate lower and the calculated K. values
would calculate lower.
The molarities of thiocyanatoiron(III) ion would calculate higher and the calculated K. values
would calculate lower.
The molarities of thiocyanatoiron(III) ion would calculate lower and the calculated K. values
would calculate higher.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

