The best fit curve line to an absorbance vs. concentration plot for standard solutions of a dye has a slope of 0.1815 mL/mg and intercept of 0.0477. A sample solution prepared of the same dye has an absorbance of 0.464 at the wavelength used to establish the Beer's law plot. What is the concentration of dye in the prepared solution? Select one: O a. 0.132 mg/mL b. 2.29 mg/mL O c. 0.464 mg/mL O d. 2.82 mg/mL e. 0.0365 mg/mL Next page
The best fit curve line to an absorbance vs. concentration plot for standard solutions of a dye has a slope of 0.1815 mL/mg and intercept of 0.0477. A sample solution prepared of the same dye has an absorbance of 0.464 at the wavelength used to establish the Beer's law plot. What is the concentration of dye in the prepared solution? Select one: O a. 0.132 mg/mL b. 2.29 mg/mL O c. 0.464 mg/mL O d. 2.82 mg/mL e. 0.0365 mg/mL Next page
Chapter25: Instruments For Optical Spectrometry
Section: Chapter Questions
Problem 25.6QAP
Related questions
Question
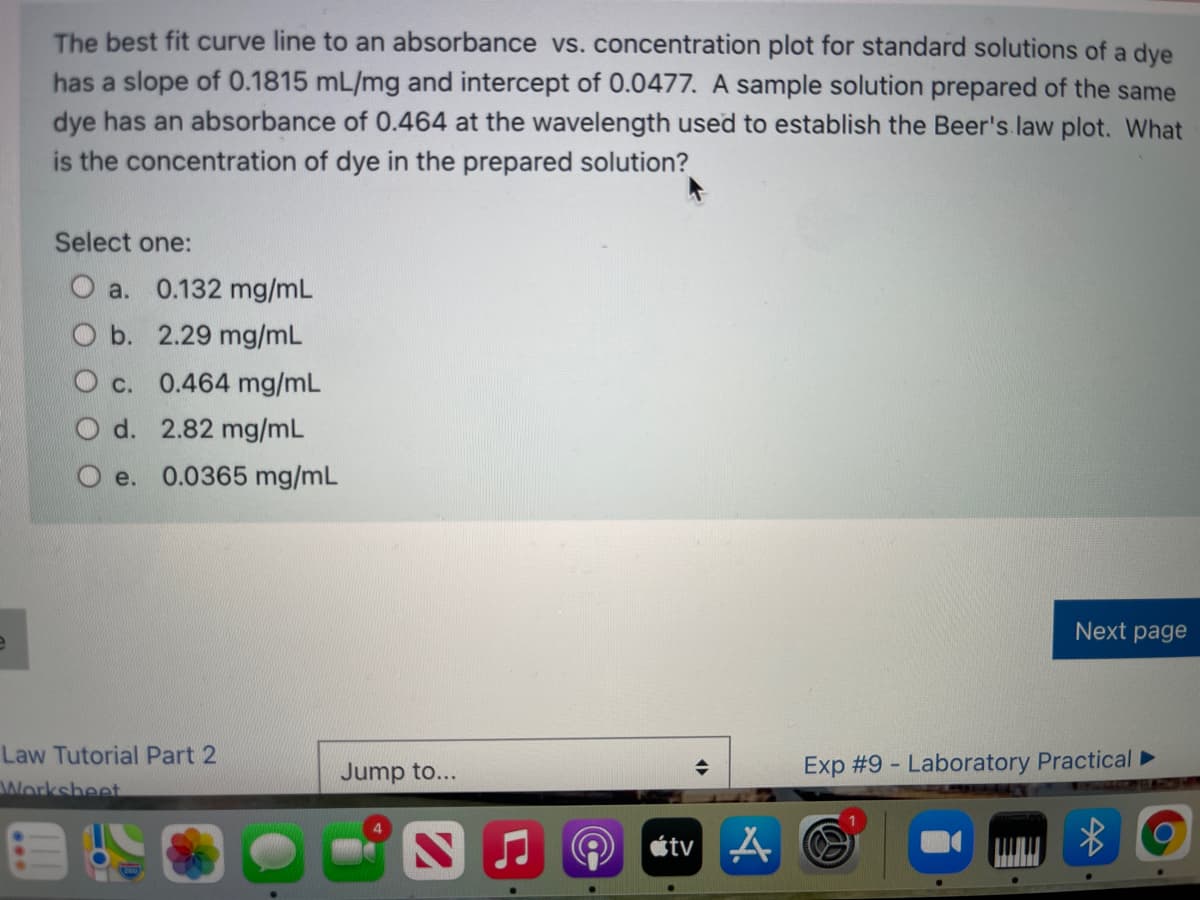
Transcribed Image Text:The best fit curve line to an absorbance vs. concentration plot for standard solutions of a dye
has a slope of 0.1815 mL/mg and intercept of 0.0477. A sample solution prepared of the same
dye has an absorbance of 0.464 at the wavelength used to establish the Beer's law plot. What
is the concentration of dye in the prepared solution?
Select one:
O a. 0.132 mg/mL
b. 2.29 mg/mL
O c. 0.464 mg/mL
O d. 2.82 mg/mL
e. 0.0365 mg/mL
Next page
Law Tutorial Part 2
Jump to...
Exp #9 - Laboratory Practical►
Worksheet
étv A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

