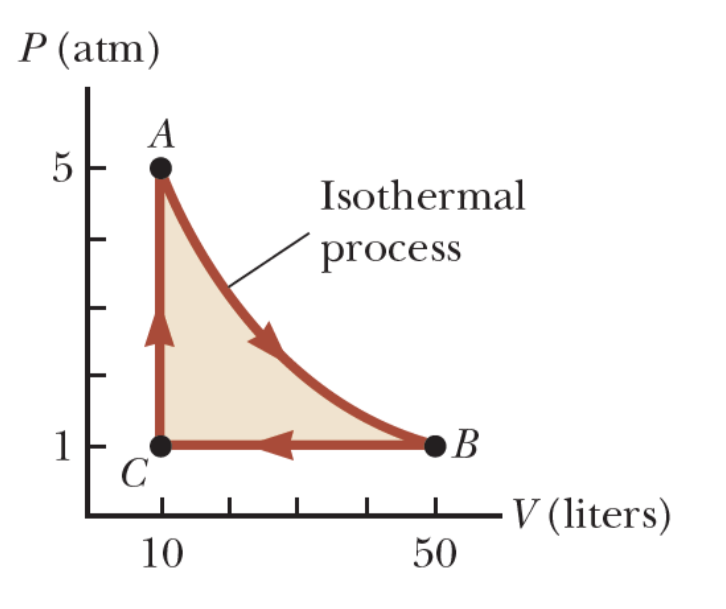
A 1.00-mol sample of an ideal monatomic gas is taken through the cycle shown in Figure. The process A → B is a reversible isothermal expansion. Calculate (a) the efficiency of the cycle and (b) explain how the efficiency compares with that of a Carnot engine operating between the same temperature extremes.
A 1.00-mol sample of an ideal monatomic gas is taken through the cycle shown in Figure. The process A → B is a reversible isothermal expansion. Calculate (a) the efficiency of the cycle and (b) explain how the efficiency compares with that of a Carnot engine operating between the same temperature extremes.
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 32P: Find the number of moles in 2.00 L of gas at 35.0 and under 7.41107 N/m2 of pressure.
Related questions
Question
A 1.00-mol sample of an ideal monatomic gas is taken through the cycle shown in Figure. The process A → B is a reversible isothermal expansion. Calculate (a) the efficiency of the cycle and (b) explain how the efficiency compares with that of a Carnot engine operating between the same temperature extremes.
Transcribed Image Text:P (atm)
A
5
Isothermal
process
В
1
C
V (liters)
10
50
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University