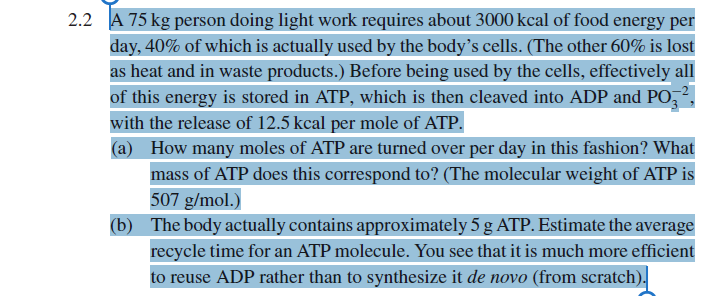
A 75 kg person doing light work requires about 3000 kcal of food energy per day, 40% of which is actually used by the body's cells. (The other 60% is lost as heat and in waste products.) Before being used by the cells, effectively all of this energy is stored in ATP, which is then cleaved into ADP and PO₂², with the release of 12.5 kcal per mole of ATP. (a) How many moles of ATP are turned over per day in this fashion? What mass of ATP does this correspond to? (The molecular weight of ATP is 507 g/mol.) (b) The body actually contains approximately 5 g ATP. Estimate the average recycle time for an ATP molecule. You see that it is much more efficient to reuse ADP rather than to synthesize it de novo (from scratch).
A 75 kg person doing light work requires about 3000 kcal of food energy per day, 40% of which is actually used by the body's cells. (The other 60% is lost as heat and in waste products.) Before being used by the cells, effectively all of this energy is stored in ATP, which is then cleaved into ADP and PO₂², with the release of 12.5 kcal per mole of ATP. (a) How many moles of ATP are turned over per day in this fashion? What mass of ATP does this correspond to? (The molecular weight of ATP is 507 g/mol.) (b) The body actually contains approximately 5 g ATP. Estimate the average recycle time for an ATP molecule. You see that it is much more efficient to reuse ADP rather than to synthesize it de novo (from scratch).
Biology 2e
2nd Edition
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:Matthew Douglas, Jung Choi, Mary Ann Clark
Chapter6: Metabolism
Section: Chapter Questions
Problem 11RQ: The energy released by the hydrolysis of ATP is primarily stored between the alpha and beta...
Related questions
Question
Biomechanics.
Transcribed Image Text:2.2 A 75 kg person doing light work requires about 3000 kcal of food energy per
day, 40% of which is actually used by the body's cells. (The other 60% is lost
as heat and in waste products.) Before being used by the cells, effectively all
of this energy is stored in ATP, which is then cleaved into ADP and PO²,
with the release of 12.5 kcal per mole of ATP.
(a) How many moles of ATP are turned over per day in this fashion? What
mass of ATP does this correspond to? (The molecular weight of ATP is
507 g/mol.)
(b) The body actually contains approximately 5 g ATP. Estimate the average
recycle time for an ATP molecule. You see that it is much more efficient
to reuse ADP rather than to synthesize it de novo (from scratch).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning