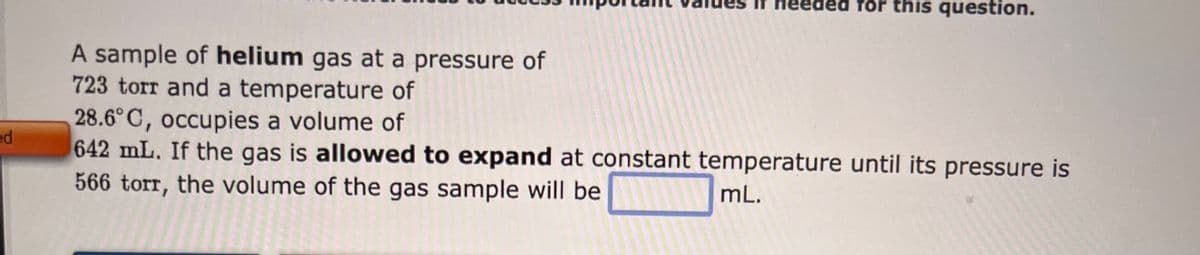
A sample of helium gas at a pressure of 723 torr and a temperature of 28.6°C, occupies a volume of 642 mL. If the gas is allowed to expand at constant temperature until its pressure is 566 torr, the volume of the gas sample will be mL.
A sample of helium gas at a pressure of 723 torr and a temperature of 28.6°C, occupies a volume of 642 mL. If the gas is allowed to expand at constant temperature until its pressure is 566 torr, the volume of the gas sample will be mL.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 31QAP: For each of the following sets of volume/temperature data, calculate the missing quantity after the...
Related questions
Question
Transcribed Image Text:ed
If needed for this question.
A sample of helium gas at a pressure of
723 torr and a temperature of
28.6°C, occupies a volume of
642 mL. If the gas is allowed to expand at constant temperature until its pressure is
566 torr, the volume of the gas sample will be
mL.

Transcribed Image Text:A sample of neon gas at a pressure of
720. torr and a temperature of
it needed for this question.
20.1°C, occupies a volume of
469 mL. If the gas is allowed to expand at constant temperature until its pressure is
480. torr, the volume of the gas sample will be
mL.
Expert Solution
Step 1
“Since you have asked multiple questions, we will solve the first question for you. If you want any specific question to be solved then please specify the question number or post only that question.”
According to the question,
Find- The final volume of the gas
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co