(a) The decomposition of a gas phase reactant, A, into gaseous products, P and Q, proceeds according to the reaction stoichiometry: A (g) (8)0 + (8) d and has been studied by measurement of the pressure increase in a constant volume system. Starting with an initial pressure of 112 torr of pure A at 713 K, the following total pressures, were measured at the times stated: Time (min) Pressure (Torr) 136 15 30 45 60 75 155 170 181 191 (i) Give a suitable expression for the integrated rate equation by which the tabulated data can be analysed to confirm that the reaction exhibits first order kinetics. (ii) Calculate the rate constant and the half life of the reaction. (ii) If the activation energy of the reaction is given by 120 kJ mol, determine the rate constant and half life at a temperature of 1 100 K.
(a) The decomposition of a gas phase reactant, A, into gaseous products, P and Q, proceeds according to the reaction stoichiometry: A (g) (8)0 + (8) d and has been studied by measurement of the pressure increase in a constant volume system. Starting with an initial pressure of 112 torr of pure A at 713 K, the following total pressures, were measured at the times stated: Time (min) Pressure (Torr) 136 15 30 45 60 75 155 170 181 191 (i) Give a suitable expression for the integrated rate equation by which the tabulated data can be analysed to confirm that the reaction exhibits first order kinetics. (ii) Calculate the rate constant and the half life of the reaction. (ii) If the activation energy of the reaction is given by 120 kJ mol, determine the rate constant and half life at a temperature of 1 100 K.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 43E: Some bacteria are resistant to the antibiotic penicillin because they produce penicillinase, an...
Related questions
Question
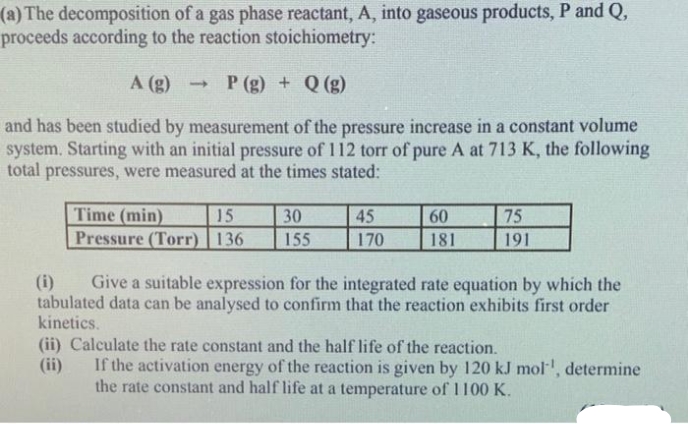
Transcribed Image Text:(a) The decomposition of a gas phase reactant, A, into gaseous products, P and Q,
proceeds according to the reaction stoichiometry:
A (g)
P (g) + Q (g)
and has been studied by measurement of the pressure increase in a constant volume
system. Starting with an initial pressure of 112 torr of pure A at 713 K, the following
total pressures, were measured at the times stated:
Time (min)
Pressure (Torr) | 136
15
30
45
60
75
155
170
181
191
(i)
Give a suitable expression for the integrated rate equation by which the
tabulated data can be analysed to confirm that the reaction exhibits first order
kinetics.
(ii) Calculate the rate constant and the half life of the reaction.
(ii)
If the activation energy of the reaction is given by 120 kJ mol, determine
the rate constant and half life at a temperature of 1100 K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning