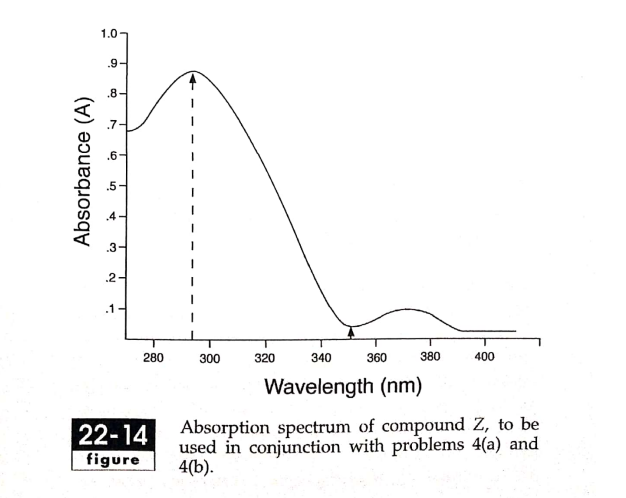
(a) What is the molar absorptivity of compound Z at 295 nm and 348nm, given the absorption spectrum shown in Fig.22-14 (which was obtained using a UV-Vis spectrophotometer and a 1mM solution of compound Z in a sample cell with a path length of 1 cm)? (b) Now you have decided to make quantitative measurement of the level of compound Z in different solutions. Based on the above spectrum, which wavelength will you use for your measurements? Give two reasons why this is the optimum wavelength.
(a) What is the molar absorptivity of compound Z at 295 nm and 348nm, given the absorption spectrum shown in Fig.22-14 (which was obtained using a UV-Vis spectrophotometer and a 1mM solution of compound Z in a sample cell with a path length of 1 cm)? (b) Now you have decided to make quantitative measurement of the level of compound Z in different solutions. Based on the above spectrum, which wavelength will you use for your measurements? Give two reasons why this is the optimum wavelength.
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.23QAP
Related questions
Question
- (a) What is the molar absorptivity of compound Z at 295 nm and 348nm, given the absorption spectrum shown in Fig.22-14 (which was obtained using a UV-Vis spectrophotometer and a 1mM solution of compound Z in a sample cell with a path length of 1 cm)?
(b) Now you have decided to make quantitative measurement of the level of compound Z in different solutions. Based on the above spectrum, which wavelength will you use for your measurements? Give two reasons why this is the optimum wavelength.
Transcribed Image Text:1.0
.9
.8-
.7-
.3
.2
.1-
280
300
320
340
360
380
400
Wavelength (nm)
22-14
figure
Absorption spectrum of compound Z, to be
used in conjunction with problems 4(a) and
4(b).
Absorbance (A)
4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
