A5 Viral inactivation is a crucial step in the purification of recombinant proteins pro- duced by mammalian cells. While microwave treatment can inactivate live viruses, it may also damage the protein product. Table P.5.2 shows the first-order inac- tivation rate constant for virus and protein, as a function of the input level of Table P.5.2 Inactivation rate constant at different energy input levels. Energy input Kyirus (1/s) Kprotein (1/s) 1 1x 10-3 0.01 x 10-3 10 5x 10-3 0.08x 10-3 100 25 x 10-3 0.62x 10-3 1000 125 x 10-3 2.08 x 10-3 10,000 625 x 10-3 1.08 x 10-3 microwave energy. Mark the level you would use, and explain why. At that level, how long of an exposure time is needed in order to reduce the virus content by 10,000-fold?
Catalysis and Enzymatic Reactions
Catalysis is the kind of chemical reaction in which the rate (speed) of a reaction is enhanced by the catalyst which is not consumed during the process of reaction and afterward it is removed when the catalyst is not used to make up the impurity in the product. The enzymatic reaction is the reaction that is catalyzed via enzymes.
Lock And Key Model
The lock-and-key model is used to describe the catalytic enzyme activity, based on the interaction between enzyme and substrate. This model considers the lock as an enzyme and the key as a substrate to explain this model. The concept of how a unique distinct key only can have the access to open a particular lock resembles how the specific substrate can only fit into the particular active site of the enzyme. This is significant in understanding the intermolecular interaction between proteins and plays a vital role in drug interaction.
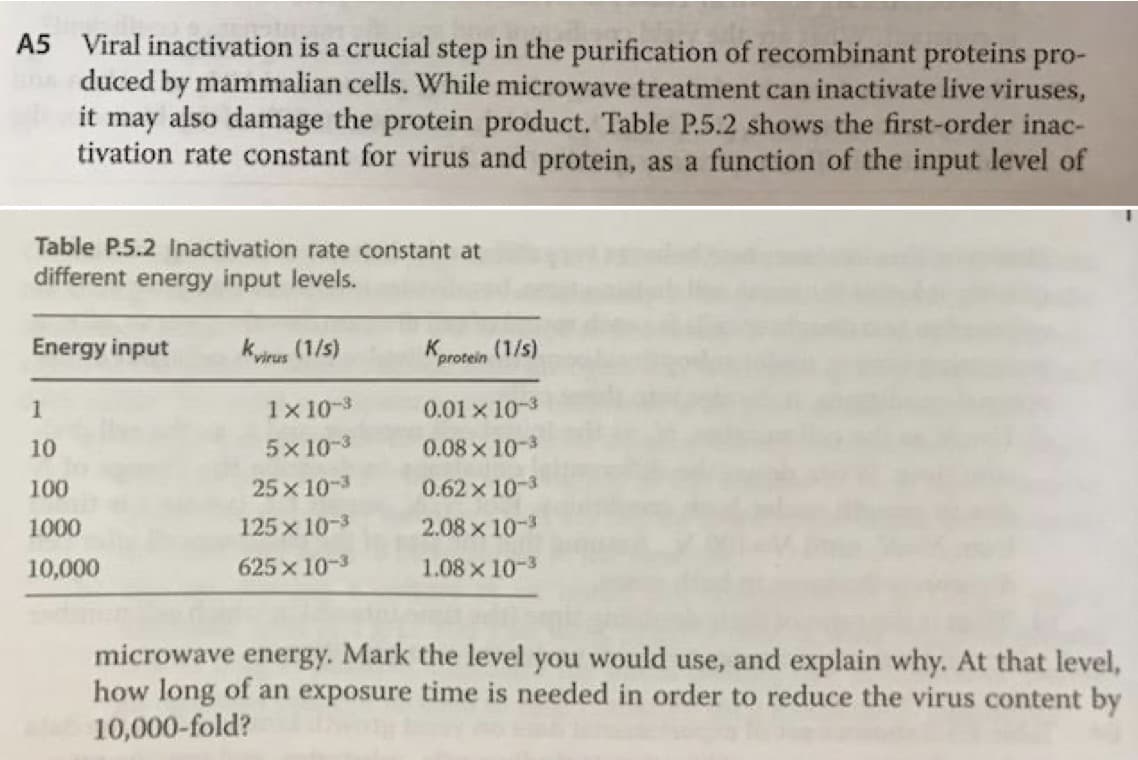
The choice of the level to be used must be done keeping in mind that the maximum viral inactivation is achieved with the minimum possible protein denaturation/inactivation.
Trending now
This is a popular solution!
Step by step
Solved in 5 steps









