t-Butyl alcohol (TBA) is an important octane enhancer that is used to replace lead additives in gasoline. TBA was produced by the liquid-phase hydration (W) of isobutene (I) over an Amberlyst-15 catalyst. The system is normally a multiphase mixture of hydrocarbon, water, and solid catalysts. The use of cosolvents or excess TBA can improve miscibility. The reaction mechanism is believed to be: K, = k,/k- W +S = W S Kw = kw/k-w W.S+1.S= TBA S+S Ke = kror/krev %3D TBA S= TBA + S KTBA = 1/KTBAD =k-TBA/KTBA Derive a rate law assuming: a) the surface reaction is rate-limiting. b) the adsorption of isobutene is limiting.
t-Butyl alcohol (TBA) is an important octane enhancer that is used to replace lead additives in gasoline. TBA was produced by the liquid-phase hydration (W) of isobutene (I) over an Amberlyst-15 catalyst. The system is normally a multiphase mixture of hydrocarbon, water, and solid catalysts. The use of cosolvents or excess TBA can improve miscibility. The reaction mechanism is believed to be: K, = k,/k- W +S = W S Kw = kw/k-w W.S+1.S= TBA S+S Ke = kror/krev %3D TBA S= TBA + S KTBA = 1/KTBAD =k-TBA/KTBA Derive a rate law assuming: a) the surface reaction is rate-limiting. b) the adsorption of isobutene is limiting.
Chapter28: Atomic Spectroscopy
Section: Chapter Questions
Problem 28.13QAP
Related questions
Question
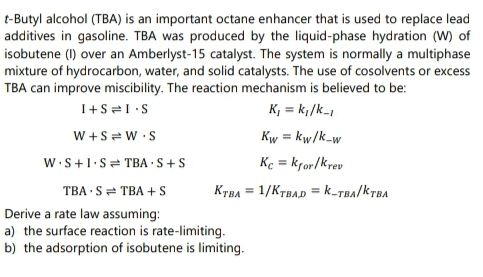
Transcribed Image Text:t-Butyl alcohol (TBA) is an important octane enhancer that is used to replace lead
additives in gasoline. TBA was produced by the liquid-phase hydration (W) of
isobutene (I) over an Amberlyst-15 catalyst. The system is normally a multiphase
mixture of hydrocarbon, water, and solid catalysts. The use of cosolvents or excess
TBA can improve miscibility. The reaction mechanism is believed to be:
K, = k;/k-
W +S = W •S
Kw = kw/k-w
W.S+1.S= TBA ·S+S
Ke = kfor/krev
%3D
TBA · S= TBA + S
KTBA = 1/KTBAD = k-TBA/KTBA
Derive a rate law assuming:
a) the surface reaction is rate-limiting.
b) the adsorption of isobutene is limiting.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole