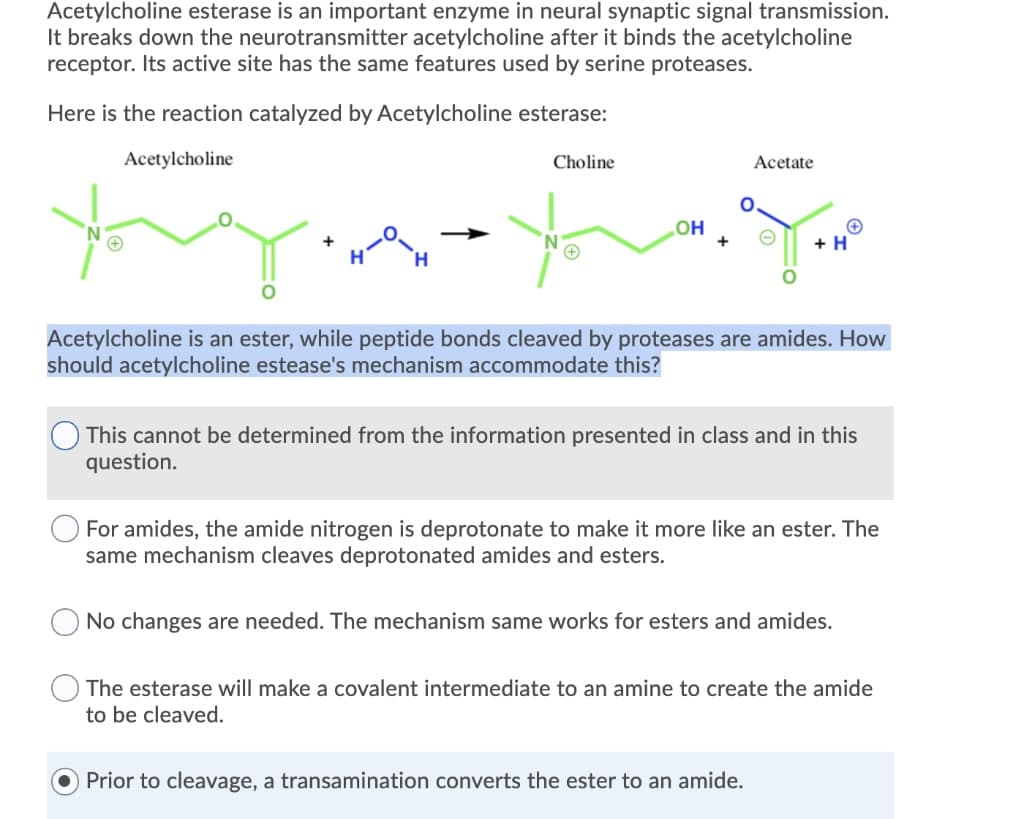
Acetylcholine esterase is an important enzyme in neural synaptic signal transmission. It breaks down the neurotransmitter acetylcholine after it binds the acetylcholine receptor. Its active site has the same features used by serine proteases. Here is the reaction catalyzed by Acetylcholine esterase: Acetylcholine + H Choline OH + Acetate + H Acetylcholine is an ester, while peptide bonds cleaved by proteases are amides. How should acetylcholine estease's mechanism accommodate this? This cannot be determined from the information presented in class and in this question. For amides, the amide nitrogen is deprotonate to make it more like an ester. The same mechanism cleaves deprotonated amides and esters. No changes are needed. The mechanism same works for esters and amides. Prior to cleavage, a transamination converts the ester to an amide. The esterase will make a covalent intermediate to an amine to create the amide to be cleaved.
Catalysis and Enzymatic Reactions
Catalysis is the kind of chemical reaction in which the rate (speed) of a reaction is enhanced by the catalyst which is not consumed during the process of reaction and afterward it is removed when the catalyst is not used to make up the impurity in the product. The enzymatic reaction is the reaction that is catalyzed via enzymes.
Lock And Key Model
The lock-and-key model is used to describe the catalytic enzyme activity, based on the interaction between enzyme and substrate. This model considers the lock as an enzyme and the key as a substrate to explain this model. The concept of how a unique distinct key only can have the access to open a particular lock resembles how the specific substrate can only fit into the particular active site of the enzyme. This is significant in understanding the intermolecular interaction between proteins and plays a vital role in drug interaction.
Step by step
Solved in 4 steps with 3 images








