ARTING AMOUNT X How many moles of CH₁2 (cyclohexane) must be reacted according to the following balanced chemical reaction to transfer -419.1 kJ of heat? ADD FACTOR g CO₂ X -46.57 C6H₁2()+ 9 O₂(g) 6 CO₂(g) + 6 H₂O(g) ΔΗ = -3919.4 kJ 1.164 x 106 32.00 mol CO₂ 9.351 0.1069 g 0₂ 6 -419.1 kJ g C6H₁2 ANSWER 6.022 x 1023 84.16 g H₂O mol C6H₁2 9 RESET mol O₂ 5 6.439 x 1022 -3919.4 mol H₂O 1 J
ARTING AMOUNT X How many moles of CH₁2 (cyclohexane) must be reacted according to the following balanced chemical reaction to transfer -419.1 kJ of heat? ADD FACTOR g CO₂ X -46.57 C6H₁2()+ 9 O₂(g) 6 CO₂(g) + 6 H₂O(g) ΔΗ = -3919.4 kJ 1.164 x 106 32.00 mol CO₂ 9.351 0.1069 g 0₂ 6 -419.1 kJ g C6H₁2 ANSWER 6.022 x 1023 84.16 g H₂O mol C6H₁2 9 RESET mol O₂ 5 6.439 x 1022 -3919.4 mol H₂O 1 J
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 47E: How many moles of isooctane must be burned to produce loo U of heat under standard state conditions?
Related questions
Question
Please help solve in this format
Transcribed Image Text:pic: Updated Schedule
C
app.101edu.co
STARTING AMOUNT
X
08
X
Aktiv Chemistry
X +
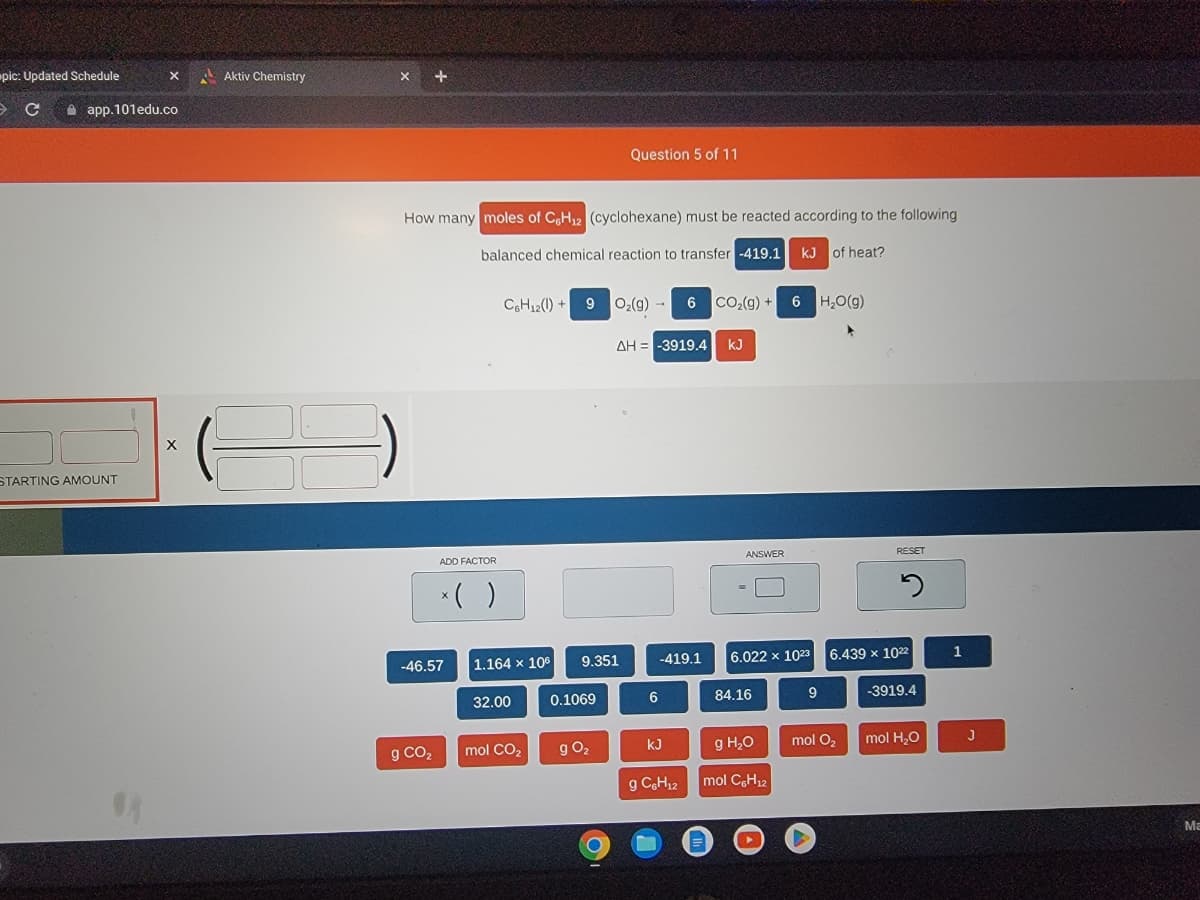
How many moles of C6H₁2 (cyclohexane) must be reacted according to the following
balanced chemical reaction to transfer -419.1 kJ of heat?
ADD FACTOR
g CO₂
x( )
-46.57
C6H12()+9
1.164 x 106
32.00
9
mol CO₂
9.351
0.1069
Question 5 of 11
g 0₂
O₂(g) → 6 CO₂(g) + 6H₂O(g)
ΔΗ = -3919.4 kJ
6
-419.1
kJ
g C6H₁2
ANSWER
6.022 x 1023
84.16
g H₂O
mol C6H₁2
9
RESET
mol O₂
5
6.439 x 1022
-3919.4
mol H₂O
1
J
Ma
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning