Ascorbic acid (MW = 176.12 g/mole) is readily oxidized by molecular iodine in the presence of acid as shown in the reaction below: %3D CH2OH CH2OH снон снон H+ + 2 HI но он Once all of the ascorbic acid has reacted with the iodine, any excess iodine will react with a starch indicator to make a purple color, indicating the endpoint. This experiment will use an ascorbic acid solution of known concentration to determine the concentration of iodine. Once that concentration is known, it will be necessary to use that solution to determine the Vitamin C content of a commercial sample, such as orange or apple juice.
Ascorbic acid (MW = 176.12 g/mole) is readily oxidized by molecular iodine in the presence of acid as shown in the reaction below: %3D CH2OH CH2OH снон снон H+ + 2 HI но он Once all of the ascorbic acid has reacted with the iodine, any excess iodine will react with a starch indicator to make a purple color, indicating the endpoint. This experiment will use an ascorbic acid solution of known concentration to determine the concentration of iodine. Once that concentration is known, it will be necessary to use that solution to determine the Vitamin C content of a commercial sample, such as orange or apple juice.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
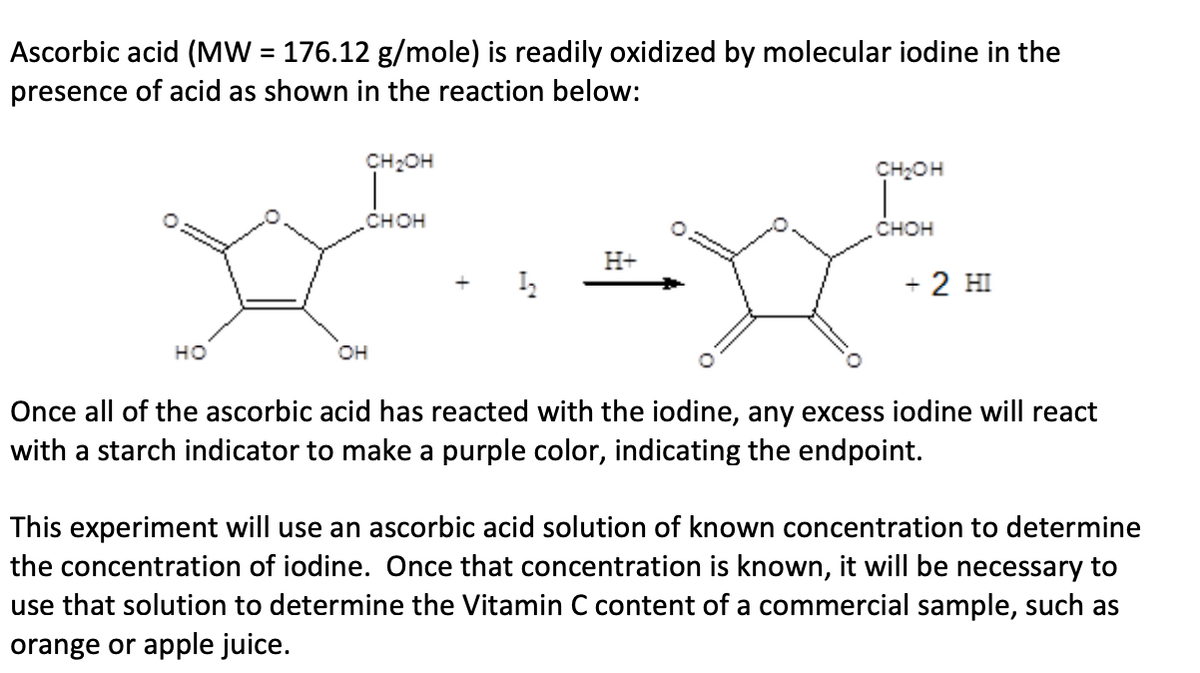
Transcribed Image Text:Ascorbic acid (MW = 176.12 g/mole) is readily oxidized by molecular iodine in the
presence of acid as shown in the reaction below:
%3D
CH2OH
CH2OH
снон
снон
H+
+ 2 HI
но
он
Once all of the ascorbic acid has reacted with the iodine, any excess iodine will react
with a starch indicator to make a purple color, indicating the endpoint.
This experiment will use an ascorbic acid solution of known concentration to determine
the concentration of iodine. Once that concentration is known, it will be necessary to
use that solution to determine the Vitamin C content of a commercial sample, such as
orange or apple juice.
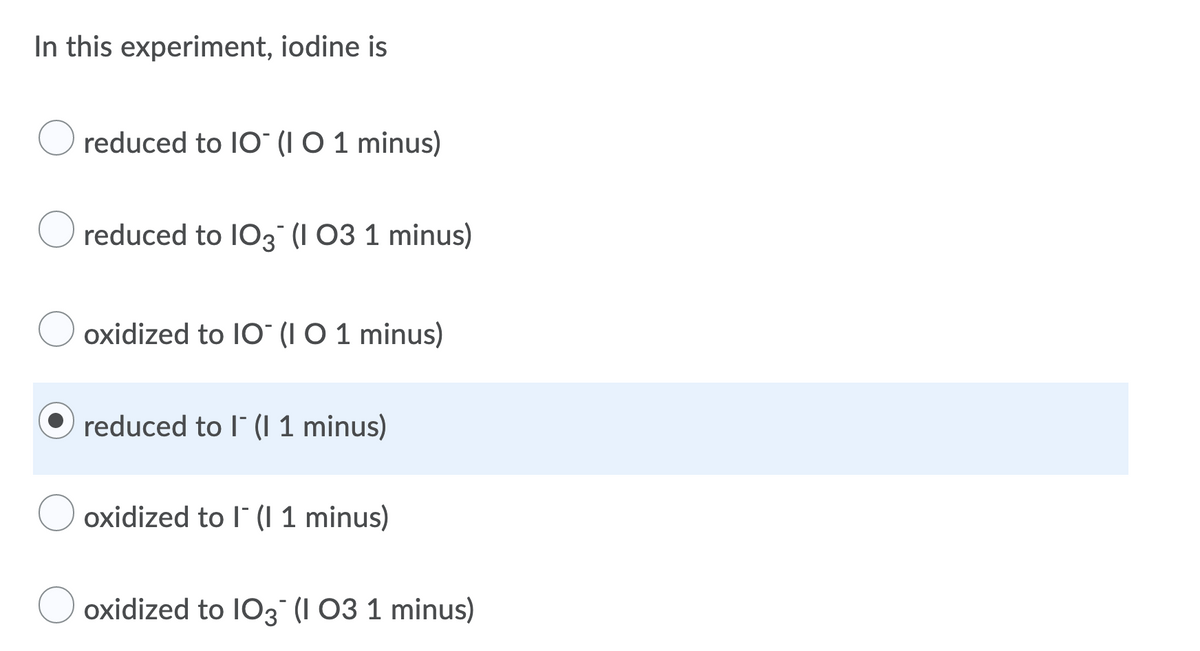
Transcribed Image Text:In this experiment, iodine is
reduced to IO (I O 1 minus)
reduced to I03 (I 03 1 minus)
oxidized to IO (I O 1 minus)
reduced to l (I 1 minus)
oxidized to l" (I 1 minus)
oxidized to IO3 (I 03 1 minus)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning