Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter13: Introduction To Symmetry In Quantum Mechanics
Section: Chapter Questions
Problem 13.11E
Related questions
Question
100%
- Based on Figure 2.13, which element has the lowest ionization energy? Does this agree with a Google search for lowest ionization energy?
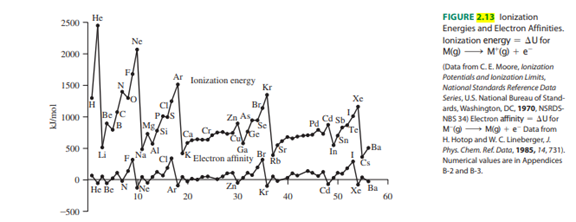
Transcribed Image Text:2500
kJ/mol
Arlonization energy
Kr
Wea
P
Zn As
Se
Mg
Si
Al
Ga
Br
Na
F
Electron affinity Rb
20
30
2000
1500
1000- Be
500-
He
0
-500
He Be
Ne
10
Kr
40
Pd
Cd Sb
In
Sn
50
Xe
Ba
Xe Ba
60
FIGURE 2.13 lonization
Energies and Electron Affinities.
lonization energy = AU for
M(g) →→→ M'(g) + e
(Data from C.E. Moore, lonization
Potentials and lonization Limits,
National Standards Reference Data
Series, U.S. National Bureau of Stand-
ards, Washington, DC, 1970, NSRDS-
NBS 34) Electron affinity- AUfor
M (g) M(g) + Data from
H. Hotop and W. C. Lineberger, J.
Phys. Chem. Ref. Data, 1985, 14, 731).
Numerical values are in Appendices
B-2 and B-3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,