Chapter3: Extraction
Section: Chapter Questions
Problem 1Q
Related questions
Question
I want the best value for percent purity and how did you arrive at the best value
Transcribed Image Text:T
F.
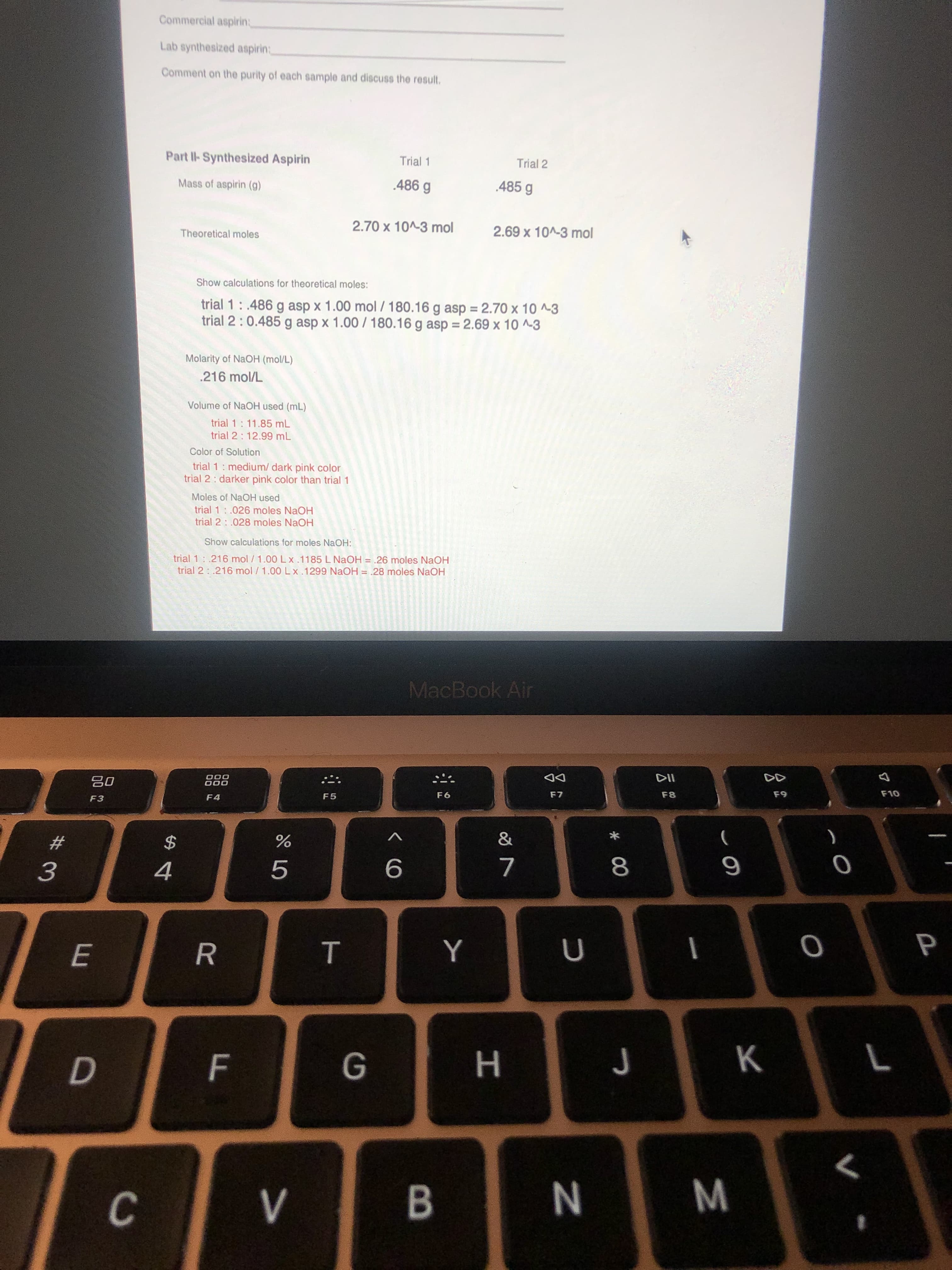
Commercial aspirinB
Lab synthesized aspirinB
Comment on the purity of each sample and discuss the result.
Part Il-Synthesized Aspirin
Trial 1
Trial 2
Mass of aspirin (g)
.486 g
485 g
2.70 x 10^-3 mol
2.69 x 10^-3 mol
Theoretical moles
Show calculations for theoretical moles:
trial 1:.486 g asp x 1.00 mol / 180.16 g asp = 2.70 x 10 ^~3
trial 2 :0.485 g asp x 1.00 / 180.16 g asp = 2.69 x 10 ^-3
Molarity of NaOH (mol/L)
.216 mol/L
Volume of NaOH used (mL)
trial 1: 11.85 mL
trial 2: 12.99 mL
Color of Solution
trial 1: medium/ dark pink color
trial 2: darker pink color than trial 1
Moles of NaOH used
trial 1:.026 moles NaOH
trial 2 : .028 moles NaOH
Show calculations for moles NaOH:
trial 1: .216 mol / 1.00 Lx.1185 L NAOH = .26 moles NaOH
trial 2: .216 mol /1.00 Lx .1299 NaOH = .28 moles NaOH
%3D
MacBook Air
000
000
DD
08
F3
F6
F5
F4
23
2$
7.
8.
3.
5.
E.
K.
H.
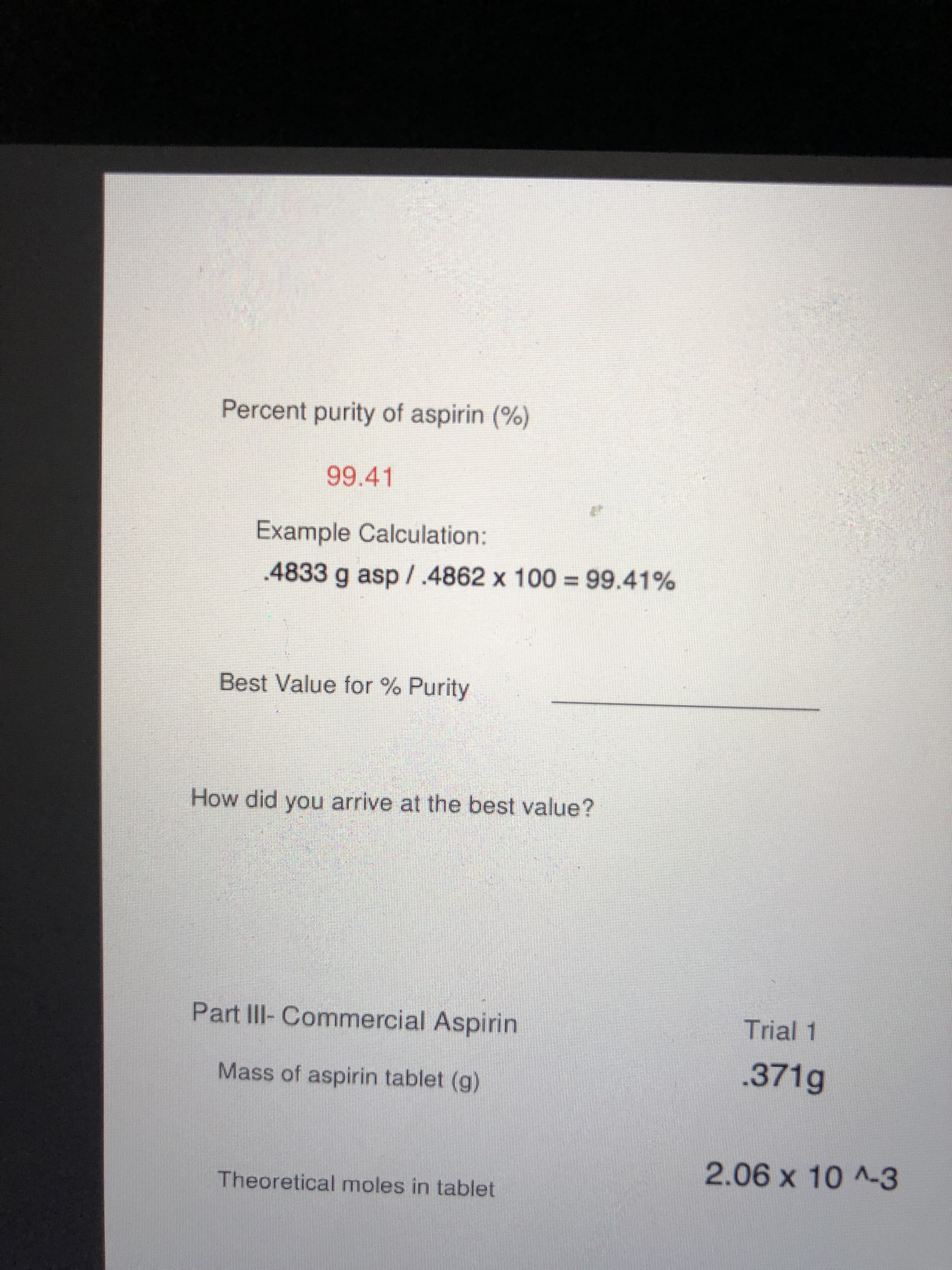
Transcribed Image Text:Percent purity of aspirin (%)
99.41
Example Calculation:
.4833 g asp/.4862 x 100 = 99.41%
Best Value for % Purity
How did you arrive at the best value?
Part III-Commercial Aspirin
Trial 1
.371g
Mass of aspirin tablet (g)
2.06x10^-3
Theoretical moles in tablet
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning