Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.16QAP
Related questions
Question
100%
Can you please explain how to calculate the equilbrium concentrations of Fe3+ and NCS-.
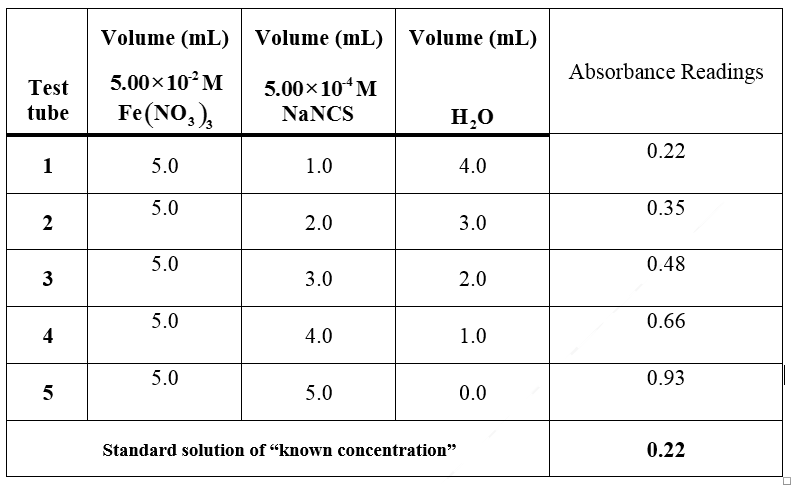
Transcribed Image Text:Volume (mL)
Volume (mL)Volume (mL)
Absorbance Readings
Test
5.00×10°M
5.00x10 M
Fe(NO,),
tube
NaNCS
H,0
0.22
1
5.0
1.0
4.0
5.0
0.35
2
2.0
3.0
5.0
0.48
3.0
2.0
5.0
0.66
4
4.0
1.0
5.0
0.93
5.0
0.0
Standard solution of "known concentration"
0.22
3.
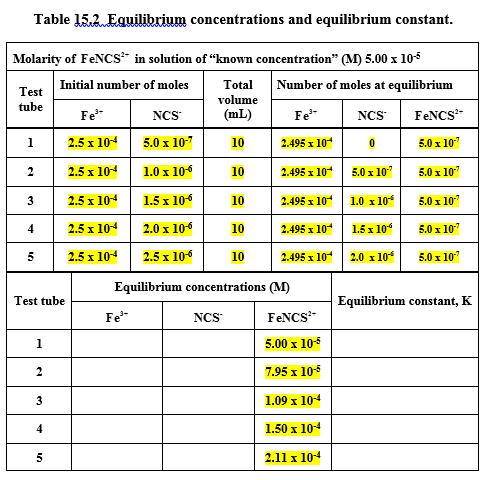
Transcribed Image Text:Table 15.2. Eguilibrium concentrations and equilibrium constant.
Molarity of FENCS* in solution of “known concentration" (M) 5.00 x 105
Initial number of moles
Total
Number of moles at equilibrium
Test
volume
(mL)
tube
Fe
NCS
Fe
FENCS
NCS
1
2.5 x 104
5.0 x 10-7
2.495 x 10
10
5.0 x 10"
2
2.5 x 104
1.0 x 10
10
2.495 x 10 5.0 x 107
5.0 x 107
3
2.5 x 104
1.5 x 106
10
2.495 x 104
1.0 x 10
5.0 x 10"
2.5 x 104
2.0 x 106
10
2.495 х 10
1.5x 10*
50х 10
5
2.5 x 104
2.5 x 106
10
2.495 x 10 2.0 x 10*
5.0 x 10"
Equilibrium concentrations (M)
Test tube
Equilibrium constant, K
Fe
NCS
FENCS
5.00 x 105
2
7.95 x 10-5
3
1.09 x 104
1.50 x 104
2.11 x 104
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,