Coal contains carbon and other elements. Carbon dioxide forms when coal burns in the presence of oxygen. Which of these is the best evidence that a chemical reaction occurs when coal burns? O the shape of the coal changes O a new substance is produced O oxygen is present O coal is made up of more than one element 11 12 13 14 15 16 17 18 19 20
Coal contains carbon and other elements. Carbon dioxide forms when coal burns in the presence of oxygen. Which of these is the best evidence that a chemical reaction occurs when coal burns? O the shape of the coal changes O a new substance is produced O oxygen is present O coal is made up of more than one element 11 12 13 14 15 16 17 18 19 20
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 7E
Related questions
Question
I need help please.
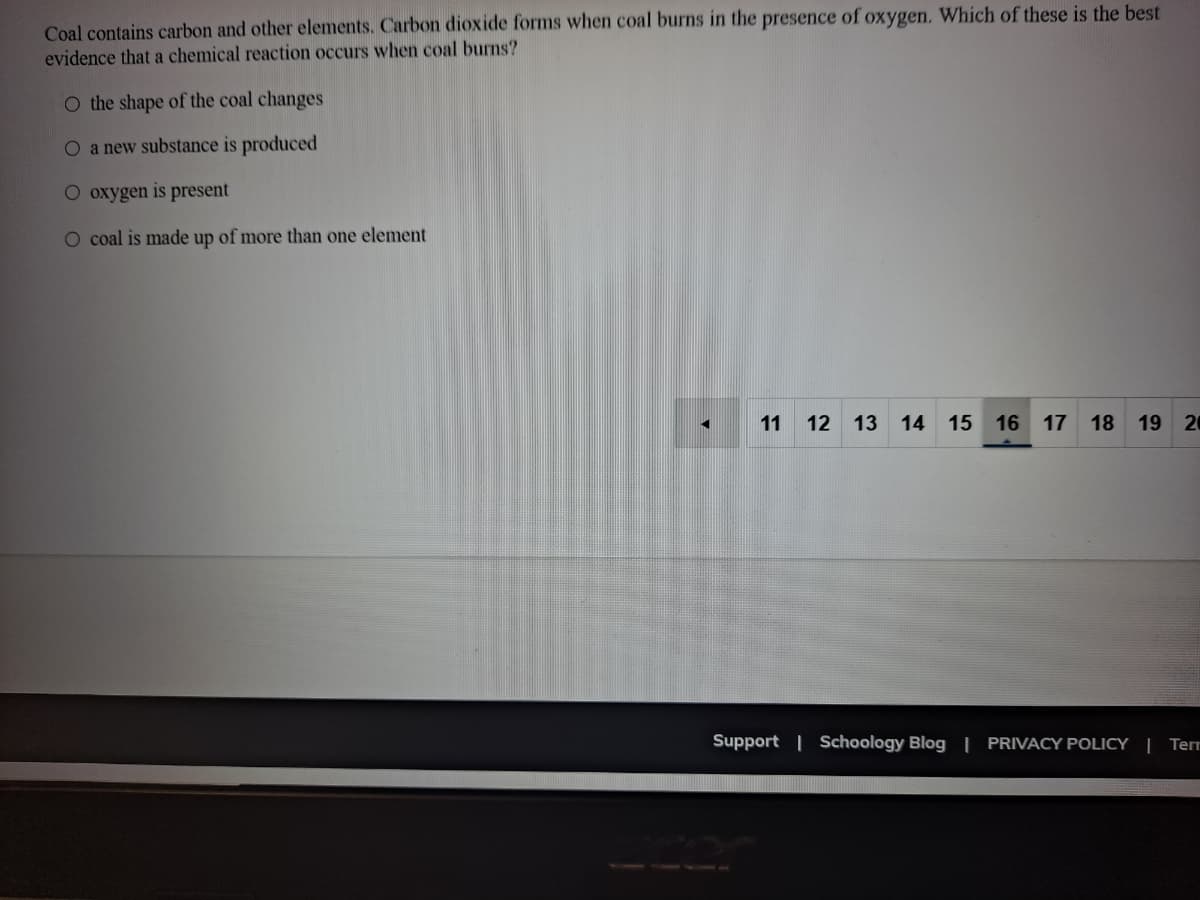
Transcribed Image Text:Coal contains carbon and other elements. Carbon dioxide forms when coal burns in the presence of oxygen. Which of these is the best
evidence that a chemical reaction occurs when coal burns?
O the shape of the coal changes
O a new substance is produced
O oxygen is present
O coal is made up of more than one element
11
12
13
14
15
16
17
18
19 20
Support | Schoology Blog | PRIVACY POLICY | Terr
Expert Solution
Step 1
When coal burn there is a chemical reaction takes place. It is a chemical reaction. It's best evidence is a new substance is produced.
So correct answer is second one
2)A new substance is formed
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning