Consider the phase diagram of substance X below. Which of the following statements is TRUE? 7.0- Solid Liquid Gas 6.0 5.0 - 4.0 D 3.0- 2.0 - 1.0 - C B 0.05 - A (atm) 200 T (K) 100 300 Select one: O a. Point D to C describes a process whereby the substance is completely vaporized. O b. The intermolecular force of attraction observed at point E is greater than that at point D. O c. At any point on the curve represented by the section ABC, the substance will exist in two phases. O d. This substance can be liquified by compression at room temperature.
Consider the phase diagram of substance X below. Which of the following statements is TRUE? 7.0- Solid Liquid Gas 6.0 5.0 - 4.0 D 3.0- 2.0 - 1.0 - C B 0.05 - A (atm) 200 T (K) 100 300 Select one: O a. Point D to C describes a process whereby the substance is completely vaporized. O b. The intermolecular force of attraction observed at point E is greater than that at point D. O c. At any point on the curve represented by the section ABC, the substance will exist in two phases. O d. This substance can be liquified by compression at room temperature.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section9.4: Solids And Changes Of Phase
Problem 9.8E
Related questions
Question
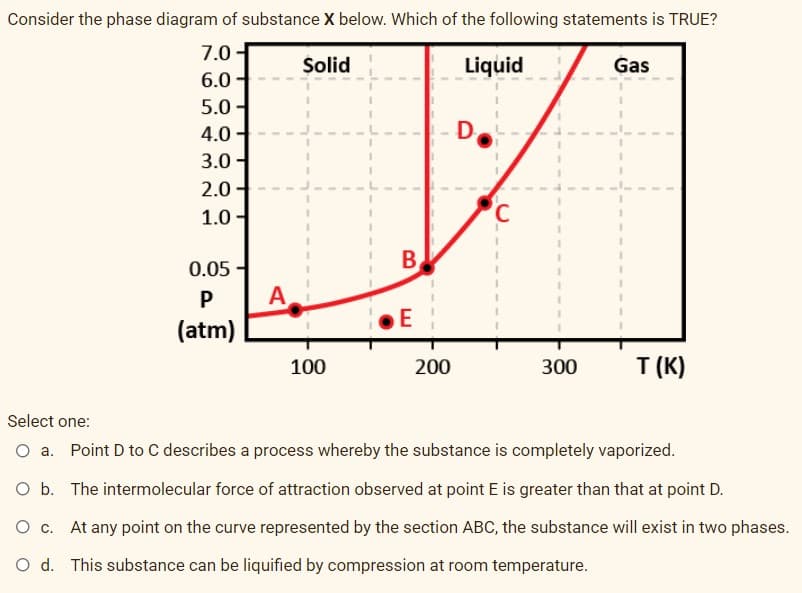
Transcribed Image Text:Consider the phase diagram of substance X below. Which of the following statements is TRUE?
7.0 -
Solid
Liquid
Gas
6.0
5.0 -
4.0
D
3.0-
2.0-
1.0 -
B
0.05 -
A
(atm)
E
200
T (K)
100
300
Select one:
O a. Point D to C describes a process whereby the substance is completely vaporized.
O b. The intermolecular force of attraction observed at point E is greater than that at point D.
O c. At any point on the curve represented by the section ABC, the substance will exist in two phases.
O d. This substance can be liquified by compression at room temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning