Enzymes are important molecules in biochemistry that catalyze reactions. The energy diagram illustrates the difference between a catalyzed reaction and an uncatalyzed reaction. Label the energy diagram. activation energy reactants (substrate) free energy reaction progress transition state uncatalyzed reaction catalyzed reaction AG for reaction products
Enzymes are important molecules in biochemistry that catalyze reactions. The energy diagram illustrates the difference between a catalyzed reaction and an uncatalyzed reaction. Label the energy diagram. activation energy reactants (substrate) free energy reaction progress transition state uncatalyzed reaction catalyzed reaction AG for reaction products
Biology (MindTap Course List)
11th Edition
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Chapter7: Energy And Metabolism
Section: Chapter Questions
Problem 11TYU: PREDICT In the following reaction series, which enzyme(s) is/are most likely to have an allosteric...
Related questions
Question
It is not 0.67 or 0.167 or 0.25.
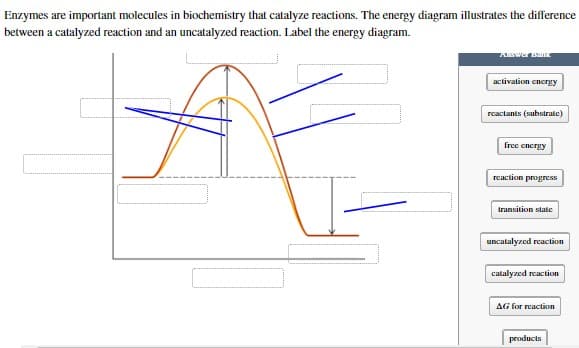
Transcribed Image Text:Enzymes are important molecules in biochemistry that catalyze reactions. The energy diagram illustrates the difference
between a catalyzed reaction and an uncatalyzed reaction. Label the energy diagram.
Answer Bank
activation energy
reactants (substrate)
free energy
reaction progress
transition state
uncatalyzed reaction
catalyzed reaction
AG for reaction
products
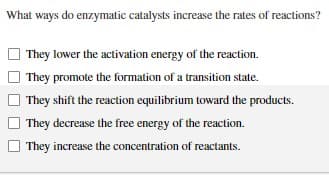
Transcribed Image Text:What ways do enzymatic catalysts increase the rates of reactions?
They lower the activation energy of the reaction.
They promote the formation of a transition state.
They shift the reaction equilibrium toward the products.
They decrease the free energy of the reaction.
They increase the concentration of reactants.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning