
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I got this question wrong on an exam and don't understand why. Could you please explain?
Transcribed Image Text:Exam 1 (...
Wha...
Sign...
Cale...
Tabl...
paci...
myL
Incorrect
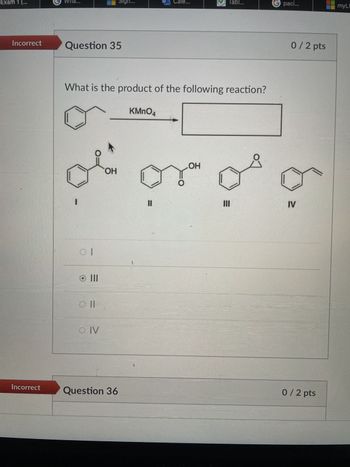
Question 35
What is the product of the following reaction?
KMnO4
0/2 pts
OH
of or of or
။
IV
III
oll
O IV
Incorrect
Question 36
0 / 2 pts
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- rsity o... ingsbo... kboard... ARED4 ethod? 7... F2 Remaining Time: 1 hour, 22 minutes, 23 seconds. Question Completion Status: A Moving to the next question prevents changes to this answer. Question 1 # The angle of the water molecule (H₂O) is 180 degrees 120 degrees 100 degrees 90 degrees 109 degree A Moving to the next question prevents changes to this answer. MAR 14 80 F3 SA $ 000 F4 tv % F5 NA MacBook Air 22 F6 ∞r F7 45.113arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardPart C: Determination of the alcohol content of unknown liquor St tiend Volume (mL) Sample ТИШТИНА 0.00% alcohol 10.0% alcohol 20.0% alcohol 40.0% alcohol 50.0% alcohol Unknown Mass (g) 1o podina ko 9.8919 9.7899 9.600 9 9.420 д % Alcohol content of the liquor_ 9.2155 9.5089 2VLELA CVOLION EAEK EVI OK DRA IVBOKY LOBAN ocen 10.00 10.00 10.00 10.00 10.00 10.00 Density (g/mL) 0.00% alcohol calculation: ися → о 0.99 во 0.99 Ino7 sm dolaW 120.00 supinubst sdi na LANG 411 be: [scordas: 0.97 0.94 in oil to notisalarisi 0.92 0.95arrow_forward
- A 1% solution of hydrochloric acid is required for the procedure. A 5% solution is available. How much of the 5% solution will be needed to make 500 mL of a 1% solution?arrow_forwardPlease helparrow_forwardAll changes save 7. When two solutions are mixed, a color change occurs. The data tables show the time between mixing and the color change for two sets of conditions. For Condition One, the solution concentrations were constant and temperature varied. For Condition Two, the temperature was constant and concentrations varied. Condition One: Concentration Time for Temperature (°C) Sample Color to Change 1 10° 36 sec 22° 14 sec Condition Two: Temperature Time for Concentration Sample Color to % Change 1. 100% 15 sec 2. 50% 24 sec Which of these statements is true according to the data? O The reaction rate is greater at 22°C than at 10°C. O Reducing the temperature increases the rate of the reaction. The reaction is affected by changes in temperature not by changes in concentration. O Decreasing the concentration increases the reaction rate. PREVIOUS 17 of 25 NEXT SAVE & EXITarrow_forward
- In a particular trial, a student got a value for R of 0.083. Calculate the percent deviation of this result. Enter a number without the % sign. The percent deviation should be reported as a positive value.arrow_forwardPlease answer questions 3 and 4 and show work please. Thank youarrow_forwardThe answer is supposed to be C though, it was a provincial question in the Chem 12 august 2005 exam. Why is it C?arrow_forward
- Below is a simple set of weights obtained while immersing potato slices in various sugar solutions over 30 minutes. I need help calculating all of this for a 30 min period a. For each solution, calculate the rate of weight change over this 30-minute period. Show your work in the space below. Once you calculate these rates - fill in the blanks below and then add these answers to your Canvas exam. Write your answer in standard notation and use 3 digits past the decimal point - e.g. 0.005, 0.030. (Note that the last zero in an answer is considered to be a digit). Show your work in the space below. 0.0 M sucrose = _______ _____________ g/min 0.4 M sucrose = _______ ____________ g/min 0.6 M sucrose = ________ ____________ g/min 0.8 M sucrose = ____________________ g/min 1.0 M sucrose = ________ __________ g/min Using the osmosis data in the question above, determine the percent weight change for each sucrose solution and fill in the blanks given below and then add these answers to…arrow_forward8 - 14 pleasearrow_forwardReal life applications of standardization of NaOH.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY