Experiment 5 Determination of the purity of KHP sample. The purity of KHP was determined by first weighing KHP using weighing by difference. and the weighed sample was titrated with NaOH solution. Calculate the % purity of the KHP sample given the following data. Express your answer in 2 decimal places. No need to include the unit. Parameter Value weight of container + KHP, g 100.2745 weight of container + remaining KHP, g 99.9679 final volume of NaOH, mL 28.66 initial volume of NaOH, mL 0.72 concentration of NaOH used, N 0.05 molar mass of KHP, g/mol 204.22 Add your answer
Experiment 5 Determination of the purity of KHP sample. The purity of KHP was determined by first weighing KHP using weighing by difference. and the weighed sample was titrated with NaOH solution. Calculate the % purity of the KHP sample given the following data. Express your answer in 2 decimal places. No need to include the unit. Parameter Value weight of container + KHP, g 100.2745 weight of container + remaining KHP, g 99.9679 final volume of NaOH, mL 28.66 initial volume of NaOH, mL 0.72 concentration of NaOH used, N 0.05 molar mass of KHP, g/mol 204.22 Add your answer
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.13QAP
Related questions
Question
The purity of KHP was determined by first weighing KHP using weighing by difference. and the weighed sample was titrated with NaOH solution. Calculate the % purity of the KHP sample given the following data. Express your answer in 2 decimal places. No need to include the unit.
I need anwer asap please show solution this is
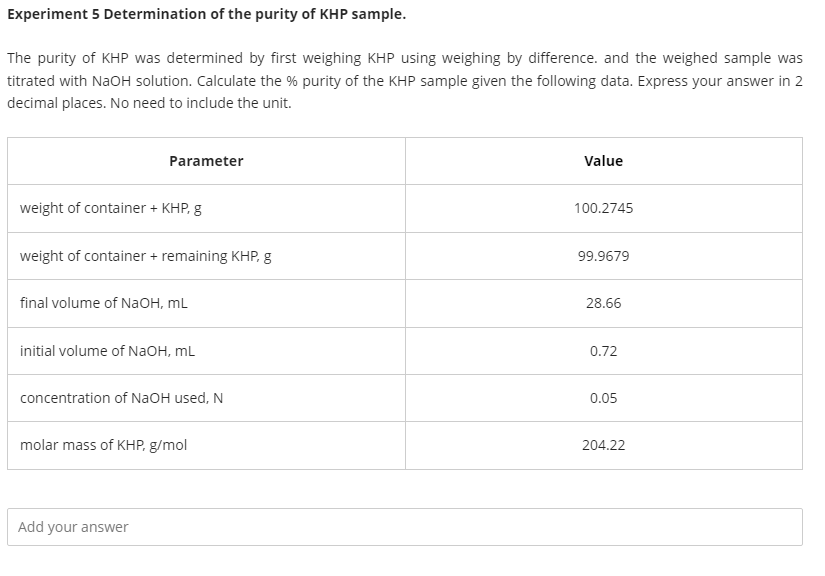
Transcribed Image Text:Experiment 5 Determination of the purity of KHP sample.
The purity of KHP was determined by first weighing KHP using weighing by difference. and the weighed sample was
titrated with NaOH solution. Calculate the % purity of the KHP sample given the following data. Express your answer in 2
decimal places. No need to include the unit.
Parameter
Value
weight of container + KHP, g
100.2745
weight of container + remaining KHP, g
99.9679
final volume of NAOH, mL
28.66
initial volume of NAOH, mL
0.72
concentration of NaOH used, N
0.05
molar mass of KHP, g/mol
204.22
Add your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



