Experiment 11: ACID-BASE TITRATION Sample Data Collection and Results Pages Volume of NaOH used in the Titration: Rough trial Trial 3 Trial 2 Trial 1 Initial reading (mL) N1.0SML o.00mL O .00m . OOm Final reading (mL) 43.15 mL 21.50mL 21.60ML 21.45ML Volume dispensed (mL) 2-1.55ML ZI.5mL 21.SOML 21.0OnL 21. o0mL Average volume of NAOH used (Trials 1-3) O. 2012 M Molarity of NaOH MOLES MOLARITY A Unknown Vinegar #_ LITERS Calculation of moles HC2H302 in 5.00 mL vinegar: 21. 00m L NaoH Mous of cetie acid
Experiment 11: ACID-BASE TITRATION Sample Data Collection and Results Pages Volume of NaOH used in the Titration: Rough trial Trial 3 Trial 2 Trial 1 Initial reading (mL) N1.0SML o.00mL O .00m . OOm Final reading (mL) 43.15 mL 21.50mL 21.60ML 21.45ML Volume dispensed (mL) 2-1.55ML ZI.5mL 21.SOML 21.0OnL 21. o0mL Average volume of NAOH used (Trials 1-3) O. 2012 M Molarity of NaOH MOLES MOLARITY A Unknown Vinegar #_ LITERS Calculation of moles HC2H302 in 5.00 mL vinegar: 21. 00m L NaoH Mous of cetie acid
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.9QAP
Related questions
Question
With the attached data, how do I calculate the mass percent of vinegar?
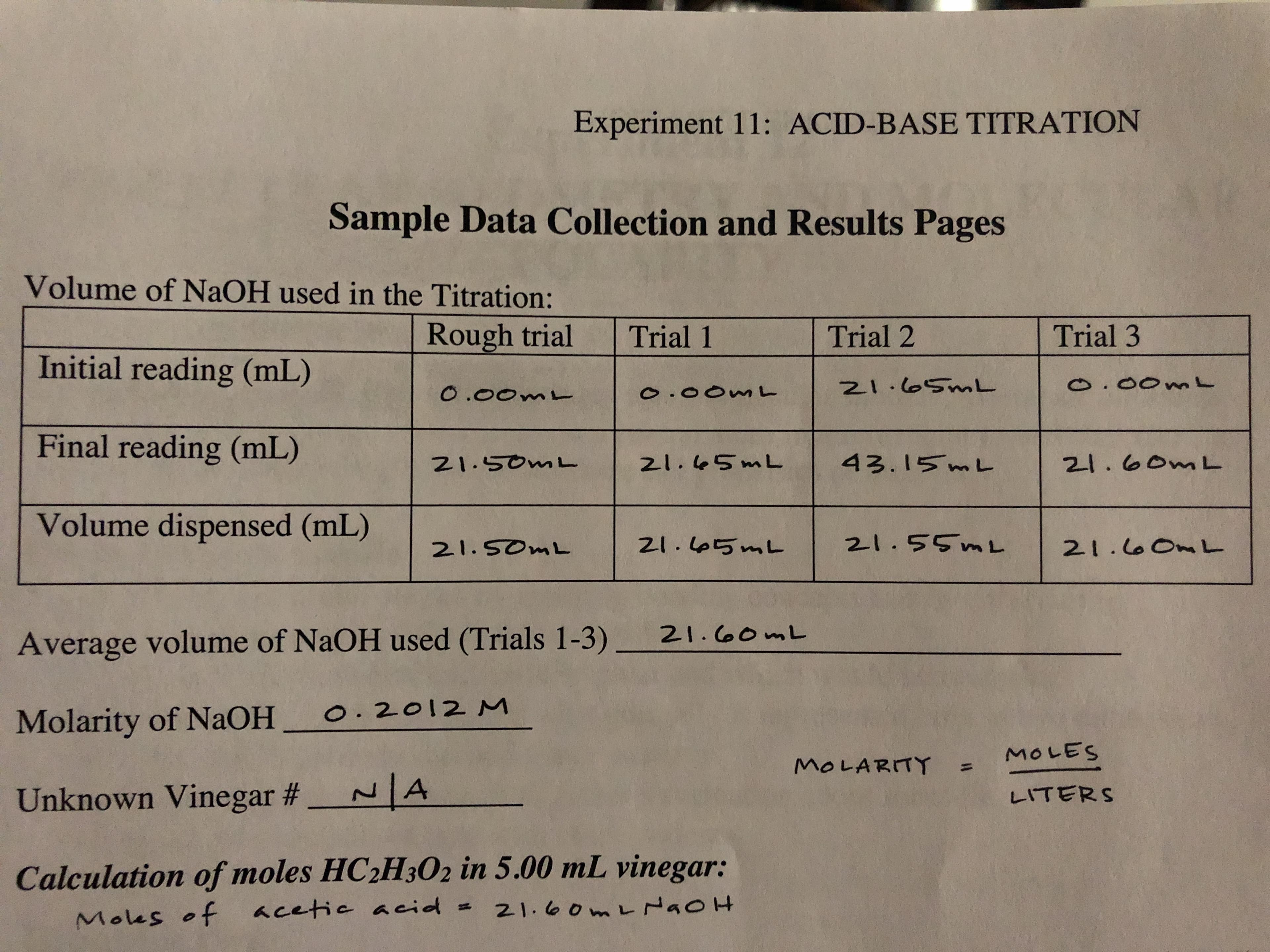
Transcribed Image Text:Experiment 11: ACID-BASE TITRATION
Sample Data Collection and Results Pages
Volume of NaOH used in the Titration:
Rough trial
Trial 3
Trial 2
Trial 1
Initial reading (mL)
N1.0SML
o.00mL
O .00m
. OOm
Final reading (mL)
43.15 mL
21.50mL
21.60ML
21.45ML
Volume dispensed (mL)
2-1.55ML
ZI.5mL
21.SOML
21.0OnL
21. o0mL
Average volume of NAOH used (Trials 1-3)
O. 2012 M
Molarity of NaOH
MOLES
MOLARITY
A
Unknown Vinegar #_
LITERS
Calculation of moles HC2H302 in 5.00 mL vinegar:
21. 00m L NaoH
Mous of cetie acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

