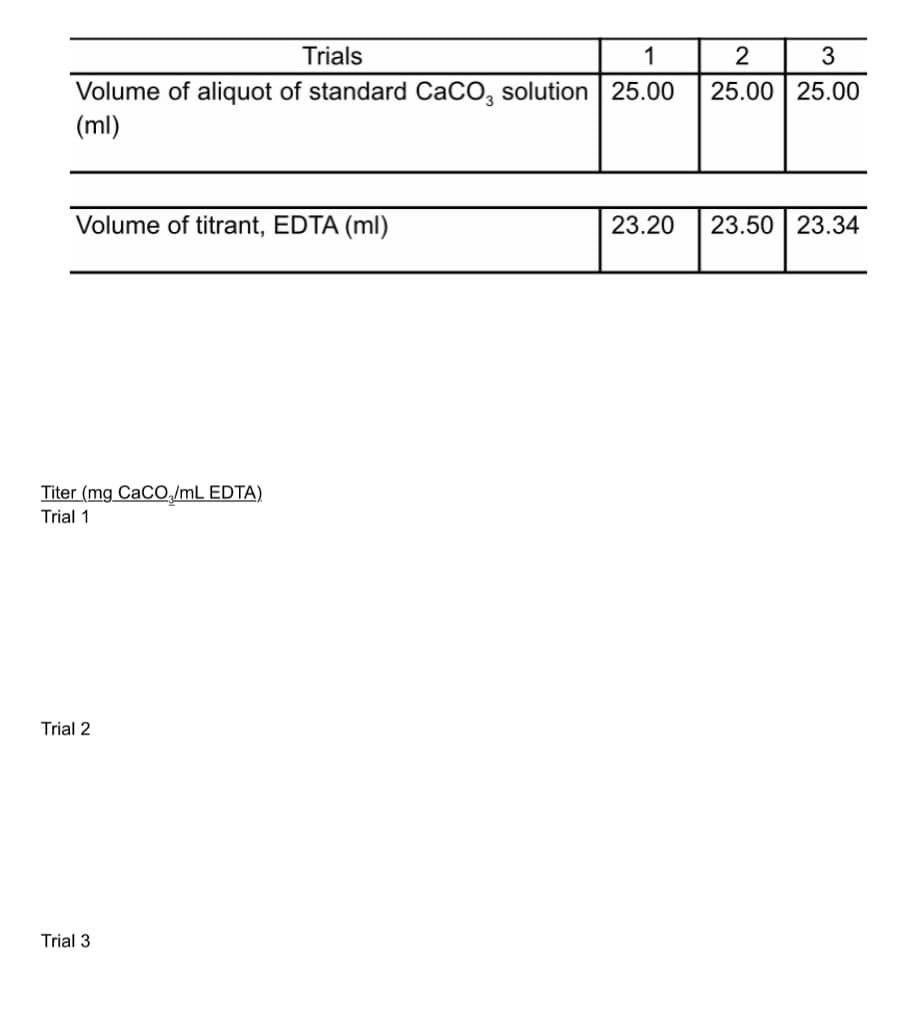
Trials 1 2 3 Volume of aliquot of standard CaCO, solution 25.00 (ml) 25.00 25.00 Volume of titrant, EDTA (ml) 23.20 23.50 23.34 Titer (mg CaCO,/mL EDTA) Trial 1 Trial 2 Trial 3
Q: Ag* 0.0000133038 Cr 0.0000133038
A: Concentration of Ag+ = 0.0000133M Concentration of Cl- = 0.0000133M
Q: The copper in an aqueous sample was determined by atomic absorption flame spectrometry. First, 10.0…
A: Here we have to plot the graph based on standard addition. Given the copper concentration of the…
Q: 300.0 mg conc 0.100 M expID: HM1-001 scale 1) EDCI, HOBT hydrate DIPEA O`HN NH H3CO HN DCM:DMF (1:2)…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: Calculate the concentration of Pd<t ion in an unknown sample based on the following data: Vol. of…
A: According to the mole concept, in terms of mass, the amount of substance in moles is equal to the…
Q: Find % error in your experiment if theoretical molarity of HC2H3O2 is 0.975 M. Show work. 5mL of…
A: For Acid-Base titrationMolarity is given
Q: 3. Internal Standard. A solution containing 3.47 mM X (analyte) and 1.72 mM S (standard) gave peak…
A: Response factor is obtained from are of analyte peak and standard peak and concentration of analyte…
Q: Determining the Ksp of Calcium Hydroxide: Ca(OH)2 (s) ↔ Ca2+ (aq) + 2OH– (aq) Ksp = [Ca2+][OH–]2…
A: From the previous question, the concentration of CaOH2 is 0.084 M. From the reaction of dissociation…
Q: A foot powder sample containing Zn was dissolved on 50.00 ml water and was titrated to the end point…
A:
Q: Hd 14 12 10 00 5 4 2 0 0 Titration Data 0.5ml Volume (ml) 1.0ml 2ml
A:
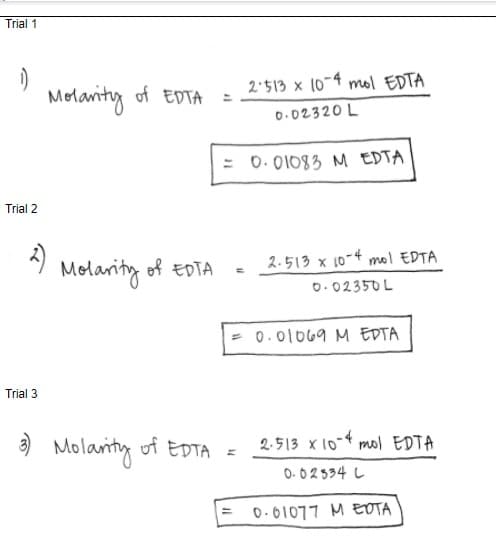
Q: Compute for the molar concentration of EDTA standardized using 50.00 mL of the CaCO3 primary…
A:
Q: Equilibrium Solutions Volume (ml) Trial # 2.00 x 10-3 M Fe(NO3)3 (ml) in 5.00 M HNO3 2.00 x…
A: Molarity is a way of expressing concentration in terms of moles of substance present per liter of…
Q: What is the result of the Analytical Method Validation of 0.1 N H2SO4 Standardization? Given: 50…
A: Introduction: Titration is the process of finding an unknown concentration of solution by using the…
Q: Istandard hard water (containing 15gms of CaCO3 per 500 mL) required 25 mL of EDTA Solution for…
A: The question is based on the concept of complexometric titrations. we determine the hardness of the…
Q: Felodipine calcium channel blocker standard (0.251mg/ml) and felodipine sample (0.245mg/ml)…
A: Given that: concentration of felodipine standard = 0.251mg/ml concentration of felodipine sample =…
Q: A water sample is approximated to have a total hardness equal to 120.0ppm. How many milliliters of…
A: By knowing the hardness amount on particular volume of solution we can calculate the millilitres of…
Q: Concentration EDTA (M): 1.94e-3 Trial #1 Trial #2 Trial #3 Volume of Water Sample titrated…
A: Given,Concentration of EDTA = 1.94 × 10-3 M = 1.94 × 10-3 mol/LVolume of water sample titrated (mL)…
Q: With the attached data, how do I calculate the number of moles of HC2H3O2 in 5.00mL of vinegar?
A: Given:Molarity of NaOH = 0.2012Volume of NaOH = 21.20 mLVolume of HC2H3O2 = 5.00 mLChemical reaction…
Q: What is the type of titration is involved this method? Direct Indirect Residual Blank 2. What is…
A:
Q: Table 6.2. Determination of hardness of water sample. Parameter Trial 1 Trial 2 volume of water…
A: For the determination of hardness we have look on data observed in experiment Here let's we assume a…
Q: A 50.00 (±0.03) mL portion of an HCl solution required 29.71(±0.03) mL of 0.01963(±0.0030) M Ba(OH)2…
A: Given, MHCl=29.71 ml×0.01963 mmol Ba(OH)2ml×2mmol HClmmol Ba(OH)250.00 ml
Q: Molar concentration of KMNO 0.02000 M 0.2189 3 7mL 0.3 mL 36.7mL Branhish Weight of complex iron…
A:
Q: In which of the following mixture(s) would CaF2(s) (Ksp = 4.0 x 10-11) form? 30 mL of 0.00020…
A:
Q: A sample of drinking water was tested for Pb+ and was found to have a Pb²* concentration of 17.46…
A: Given the concentration of the unspiked sample, Cunspiked = 17.46 ppb Now 1.00 mL of 2.29 ppb Pb2+…
Q: Question 7 A student performed three DCM layer titrations in Part 2 and they reported the following…
A:
Q: 4. (review: multi-point standard addition method) Calcium is determined in river water sample using…
A:
Q: Titration Heating Volume of DCIP (ml) Average Amt of Conc of #3 time vol of Vit C in Vit C Sample…
A: Ascorbic acid in Vitamin C reacts with DCIP in 1:1 fashion.
Q: A foot powder sample containing Zn was dissolved on 200.00 mL water and was titrated to the end…
A: The question is based on the concept of chemical equilibrium. we have to calculate pZn value for the…
Q: Standard Solution 2 3 4 6. Conc. Of PO, 3- (ppm) 0.00 0.2 0.4 0.6 0.8 1.0 Absorbance 0.00 0.033…
A: A question based on Beer-Lambert law, which is to be accomplished.
Q: Calculate Average Molarity of EDTA and Relative Average Deviation (R.A.D.)
A: According to Law of Chemical Equivalence M1V1 = M2V2 M1 = molarity of EDTA = M2V2/V1 Trial…
Q: Table 8.1 Hardness determination of water samples. mL of Volume of NazH2Y 2 H2O, mL Ca2+, mg/L…
A: First you have to standardize the EDTA solution before the titration of hard sample solution. Let…
Q: Given the following data Dissolved Oxygen Titration Data Trial 1 Preparation of titrant 0.4455 g…
A:
Q: The detecton limits reported for Al with ICP-AES and GF-AAS are 2 ppb and 5 picograms, respectivly.…
A: Given:Al with ICP-AES = 2 ppb = 2 µg/L = 2×10-6 g/L.Mass of Al with GF-AAS = 5 picogram = 5×10-12…
Q: Volume vs pH 14 12 10 equivalence point 2 equivoler ce point - 10 15 20 25 30 Volume of base added…
A: As equivalence point is at pH 8 it means we have weak acid assume HA . So salt of weak acid have A-…
Q: A foot powder sample containing Zn was dissolved on 200.00 mL water and was titrated to the end…
A:
Q: With the attached data, how do I calculate the mass percent of vinegar?
A:
Q: Standardization of EDTA Solution Weight of pure CaCO3: 0.2517 g % Purity of CaCO3: 98.0% Total…
A: The experiment data given is, Trial 1 2 3 The volume of standard CaCO3 solution (ml) 25.00…
Q: i need help calculating the molar concentration for part C
A:
Q: Trials 1 2 Volume of aliquot of standard CaCO, solution 25.00 25.00 25.00 (ml) Volume of titrant,…
A: Given ; Volume of CaCO3 solution = 25.00 mL = 0.02500 L (since 1 L =…
Q: In the next part of the experiment, you used the standard solution of sodium oxalate to standardize…
A: The data given is,
Q: Principle: lodometry The percentage purity of cupric sulfate set by the NF is 98.5 to 100.5%…
A: Given: Concentration of sodium thiosulfate = 0.1 N
Q: Volume HCl (mL) 25.00 а. 0.00 b. Initial buret reading (mL) 25.40 c. Final buret reading (mL) 25.40…
A: Equation NaOH(aq) + HCl (aq) ——> NaCl (aq) + H2O(l) Molarity = no. of moles / volume (Lt)
Q: Chosen wavelength : 400 nm EDTA Volume (mL) Absorbance O mL 0.445 1 mL 0.439 2 ml 0.434 3 ml 0.428 4…
A: Answer
Q: A foot powder sample containing Zn was dissolved on 100.00 mL water and was titrated to the end…
A: The question is based on the concept of complexometric titrations. We have to calculate P value of…
Q: Data & Results: Complete the table. TRIAL 1 TRIAL 2 TRIAL 3 Wt. of vinegar sample Vol. of vinegar…
A: % CH3COOH = Normality of NaOH * Volume of NaOH * Eq wt of CH3COOH * 100 / Weight of sample
Q: solubility
A: Borax : Na2B Na2B <-------> 2Na+(aq) + B^2-(aq) At Equilibrium : [Na+] = 2s and [B^2-] = s…
Q: Prepare a standard curve from the data below from a Bradford assay. Samples were prepared from a…
A: Use the conversion factor 1000 μg1 mg to convert amount of protein in mg to μg. Example: 2.5 mg…
Q: Initial burette reading (mL) 2.29 1.41 1.95 Molarity of NaOH (M) 0.100 0.100 0.100…
A:
Q: Formula: ml base x N x meq. wt. x 100 %w/w = Sample weight A 4.59 ml sample of HCI, specific gravity…
A: 50.5ml of 0.9544N NaOH is required. HCl and NaOH reacts in 1:1 ratio. 4.59ml of sample was used…
Q: Standardization of EDTA Solution Weight of pure CaCO3: 0.2514 g % Purity of CaCO3: 98.0% Total…
A: Molarity of a solution is defined as the number of moles of solute present in one litre of a…
Solve for titer. Pls show me the solution or step by step process so I can follow. Thank you
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

- Standardization of EDTA Solution Weight of pure CaCO3: 0.2517 g % Purity of CaCO3: 98.0% Total volume of standard CaCO3 solution: 250.00 mL Trial 1 2 3 Volume of standard CaCO3 solution (ml) 25.00 25.00 25.00 Final Volume Reading EDTA (ml) 24.30 47.95 24.72 Initial Volume Reading EDTA (ml) 0.00 24.30 0.20 Volume of titrant, EDTA (ml) Molarity of EDTA (M)Standardization of EDTA Solution Weight of pure CaCO3: 0.2514 g % Purity of CaCO3: 98.0% Total volume of standard CaCO3 solution: 250.00 mL Using the information given in the table, show a step-by-step process to compute the missing values. Take note of the % purity and total volume. Trial 1 2 3 Volume of standard CaCO3 solution (ml) 25.00 25.00 25.00 Final Volume Reading EDTA (ml) 24.30 47.95 24.72 Initial Volume Reading EDTA (ml) 0.00 24.30 0.20 Volume of titrant, EDTA (ml) Molarity of EDTA (M)Density of solution:Trial 1: 1.2 g/mLTrial 2: 1.2 g/mLTrial 3: 1.2 g/mL Average density = 1.2 g/mL What is the relative average deviaion, %?
- Trial 1 Trial 2 Trial 3 Initial burette reading (mL) 2.29 1.41 1.95 Molarity of NaOH (M) 0.100 0.100 0.100 Volume of vinegar sample (mL) 5.00 5.00 5.00 Final burette reading (mL) 50.37 49.39 49.84 Table 2. Titration data Trial 1 Trial 2 Trial 3 Initial burette reading (mL) 2.29 1.41 1.95 Molarity of NaOH (M) 0.100 0.100 0.100 Volume of vinegar sample (mL) 5.00 5.00 5.00 Final burette reading (mL) 50.37 49.39 49.84 Expected color at end point Volume of NaOH used (mL) 48.08 47.98 47.89 Compute for the ff: a. Average moles of acetic acid (mol)? b. Average molarity of acetic acid (M)? c. Average molarity of acetic acid (M)?A 50.00 (±0.03) mL portion of an HCl solution required 29.71(±0.03) mL of 0.01963(±0.0030) M Ba(OH)2 to reach an end point with bromocresol green indicator. The molar concentration of the HCl is calculated using the equation below (attached image): a.) Calculate the uncertainty of the result (absolute error). M=0.02333(±?????) M b.) Calculate the coefficient of variation for the result. CV= (Sy/y) x 100%Felodipine calcium channel blocker standard (0.251mg/ml) and felodipine sample (0.245mg/ml) solutions were prepared and injected to the HPLC. The peak area of felodipine standard is 275428 and the sample is 272982. The potency (purity) of felodipine standard is 98.9%. What is the assay percentage of felodipine? a. 102.23 b. 98.71 c. 101.07 d. 100.42
- The internal standard method compensates for errors that affect both the analyte and the reference. True False20.0 mL water sample was titrated with 0.0100 M EDTA solution that required 14.50 mL for CaCO3 hardness. The total hardness of the water sample in terms of CaCO3 (MW = 100.1 g/mole) in parts per million is _________? Note: Express final answer using least number of significant figures. 2. If 35.2 mL of EDTA solution is required to reach the endpoint with 0.3120-g of primary standard grade CaCO3 (MW = 100.1 g/mole), the normal concentration of the EDTA (MW = 292.24) solution is _________ N?FT-IR technique can be utilized for the analysis of unknown analytes by matching it with__________. Both choices are correct Commercial library/database Reference standard
- Which sentence is false about gravimetric analysis? a. It is used for inorganic b. It is used to assay barium c. It is used to assay of d. Relative precision 3% to 4%I got a task in analytical chemistry to calibrate, standardize, verify and validate an analytical balancesA volume of 250 ml of a 0.05 M solution of a reagent of formula weight (relative molecular mass) 40 was made up, the weighing being done by difference. The standard deviation of each weighing was 0.0001 g. The standard deviation of the volume of solvent used was 0.05 ml. Express this as a relative standard deviation. Hence calculate the relative standard deviation of the molarity of the solution.

