For the following reactions, predict whether the mole fraction of the reactants or products increases or remains the same when the volume of the reaction vessel is increased. SO2 (9) + 1/2 0, (9) = SO3 (9) а. mole fraction of reactants increases mole fraction of products increases mole fraction remains the same 2 CH4 (9) = C,H2 (g) + 3 H2 (g) b. Omole fraction of reactants increases mole fraction of products increases O mole fraction remains the same N2(g) + 2 O2 (9) = 2 NO2(9) c. Omole fraction of reactants increases mole fraction of products increases mole fraction remains the same
For the following reactions, predict whether the mole fraction of the reactants or products increases or remains the same when the volume of the reaction vessel is increased. SO2 (9) + 1/2 0, (9) = SO3 (9) а. mole fraction of reactants increases mole fraction of products increases mole fraction remains the same 2 CH4 (9) = C,H2 (g) + 3 H2 (g) b. Omole fraction of reactants increases mole fraction of products increases O mole fraction remains the same N2(g) + 2 O2 (9) = 2 NO2(9) c. Omole fraction of reactants increases mole fraction of products increases mole fraction remains the same
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 102QRT
Related questions
Question
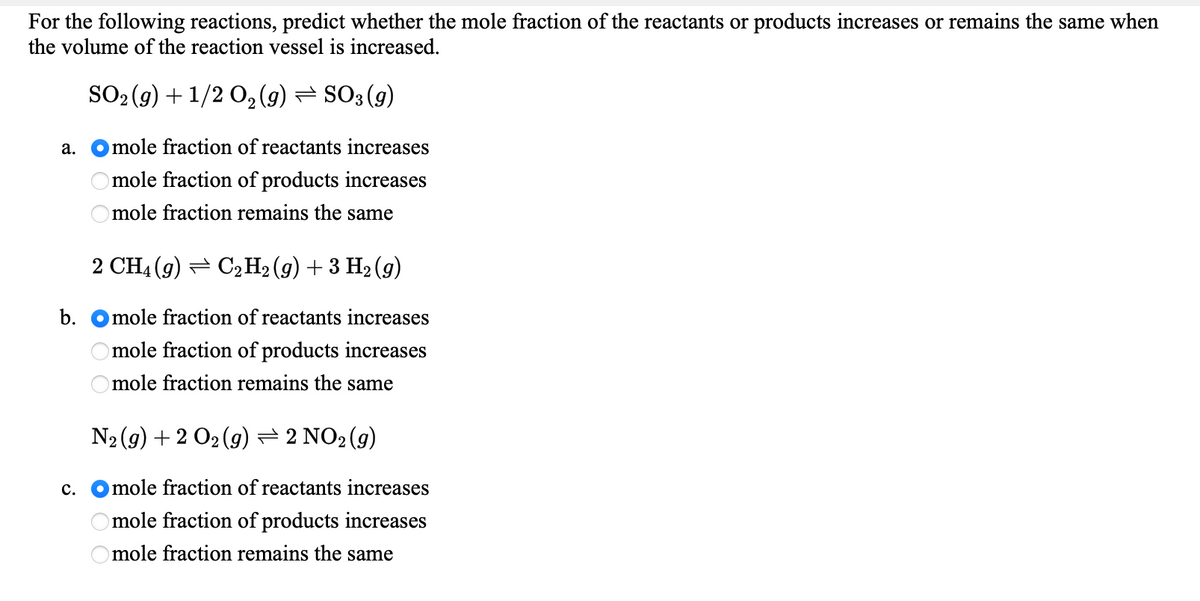
Transcribed Image Text:For the following reactions, predict whether the mole fraction of the reactants or products increases or remains the same when
the volume of the reaction vessel is increased.
SO2 (9) + 1/2 0, (9) = SO3 (g)
а.
mole fraction of reactants increases
mole fraction of products increases
mole fraction remains the same
2 CH4 (g) = C2H2 (g) + 3 H2 (g)
b.
mole fraction of reactants increases
mole fraction of products increases
Omole fraction remains the same
N2 (9) + 2 O2 (9)=2 NO2 (g)
с.
mole fraction of reactants increases
mole fraction of products increases
Omole fraction remains the same
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning