Full Adaptive Learning Objectives Mastery POWERED BY KNEWTON 1 2 3 2 Learning Objective: 8.C Quantify mass and number of moles in solutions given their concentrations. Adaptive Assignment O See page 336 Question 1st attempt Jl See Periodic Table O See Hint A concentrated solution of HF has a molarity of 9.75 M. You add 31.00 mL to a flask and then dilute with water. The mass of the final solution is 125.0g. What is the mass percent of HF? < 12 > A SUBMIT ANSWER MacBook Air 1421 888 F4 FS F6 F7 FO 19 $10 F3
Full Adaptive Learning Objectives Mastery POWERED BY KNEWTON 1 2 3 2 Learning Objective: 8.C Quantify mass and number of moles in solutions given their concentrations. Adaptive Assignment O See page 336 Question 1st attempt Jl See Periodic Table O See Hint A concentrated solution of HF has a molarity of 9.75 M. You add 31.00 mL to a flask and then dilute with water. The mass of the final solution is 125.0g. What is the mass percent of HF? < 12 > A SUBMIT ANSWER MacBook Air 1421 888 F4 FS F6 F7 FO 19 $10 F3
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 9ALQ: onsider separate 100.0-g samples of each of the following: NH3 , N2O , N2H4 , HCN , HNO3 . Arrange...
Related questions
Question
100%
Transcribed Image Text:THIS Webpage is using significant memory. Closing it may improve the responsiveness of your Mac.
< Adaptive Chapter 8 A
li 47%
画04/11/21
mmorr197@students.kennesa
SCORE
Full Adaptive
O KNEWTON
POWERED BY
Learning Objectives Mastery "
1 2
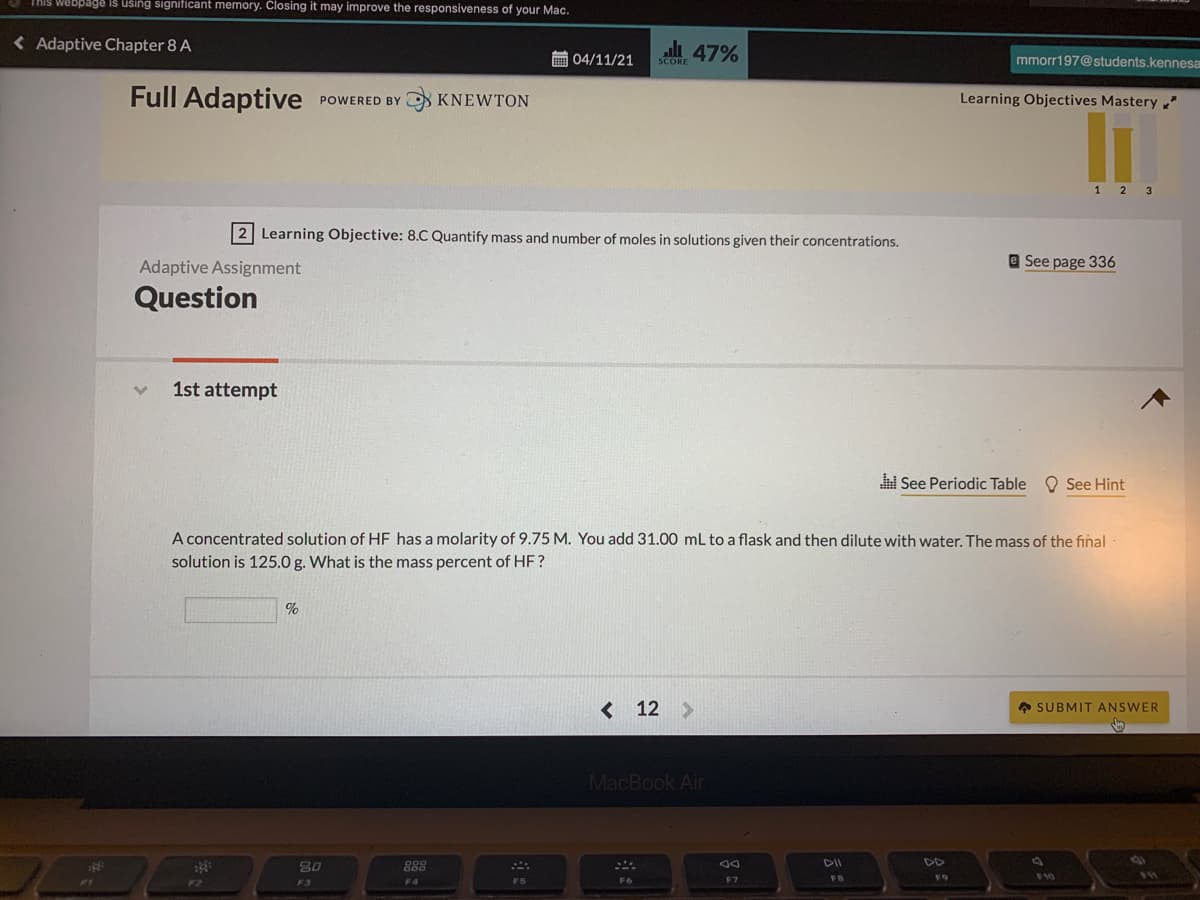
2 Learning Objective: 8.C Quantify mass and number of moles in solutions given their concentrations.
Adaptive Assignment
e See page 336
Question
1st attempt
i See Periodic Table O See Hint
A concentrated solution of HF has a molarity of 9.75 M. You add 31.00 mL to a flask and then dilute with water. The mass of the final
solution is 125.0 g. What is the mass percent of HF?
%
< 12
* SUBMIT ANSWER
MacBook Air
888
DD
$10
F3
F2
F4
F5
F6
F7
FB
F9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning