(ii) Calculate the free energy change at standard conditions for the following reaction: Acetaldehyde + NADH + H* + Ethanol + NAD The half- reactions are: Acetaldehyde + 2H + 2e Ethanol NAD + 2H + 2e - NADH + H- E"= - 0.20V E"= - 0.32V (F= 96.485 kJ/V/mol)
(ii) Calculate the free energy change at standard conditions for the following reaction: Acetaldehyde + NADH + H* + Ethanol + NAD The half- reactions are: Acetaldehyde + 2H + 2e Ethanol NAD + 2H + 2e - NADH + H- E"= - 0.20V E"= - 0.32V (F= 96.485 kJ/V/mol)
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter18: Glycolysis
Section: Chapter Questions
Problem 17P
Related questions
Question
answer ii only
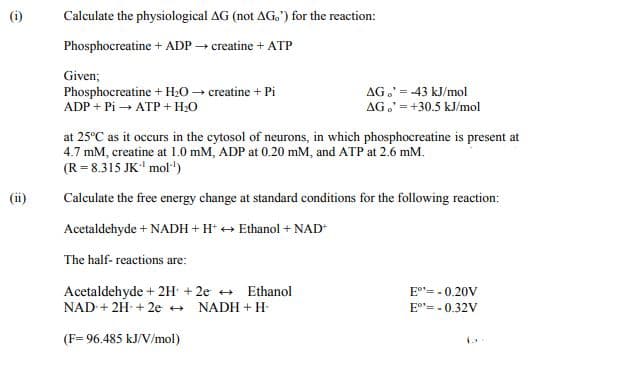
Transcribed Image Text:(i)
Calculate the physiological AG (not AG.') for the reaction:
Phosphocreatine + ADP creatine + ATP
Given;
Phosphocreatine + H20 → creatine + Pi
ADP + Pi → ATP + H:0
AG,' = -43 kJ/mol
AG.' =+30.5 kJ/mol
at 25°C as it occurs in the cytosol of neurons, in which phosphocreatine is present at
4.7 mM, creatine at 1.0 mM, ADP at 0.20 mM, and ATP at 2.6 mM.
(R= 8.315 JK' mol"')
(ii)
Calculate the free energy change at standard conditions for the following reaction:
Acetaldehyde + NADH + H* + Ethanol + NAD
The half- reactions are:
Acetaldehyde + 2H + 2e + Ethanol
NAD + 2H + 2e +
E"= - 0.20V
NADH + H-
E°"= - 0.32V
(F= 96.485 kJ/V/mol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning