In the experiment, the amount of acetic acid in vinegar was determined by titrating with 0.1 M NaOH solution. In the analysis, three trials were performed to obtain more precise result. The table shows the initial and final burette readings for three titrations: Initial Burette Reading (mL) Final Burette Reading (mL) Trial 1 2.9 10.6 Trial 2 10.6 18.1 Trial 3 18.1 25.7 According to the given data, calculate molarity of acetic acid in the commercial vinegar. Please be sure that your answer includes followings; a) moles of NaOH for each trial b) average mole of NaOH c) average mole of acetic acid in 50 mL solution
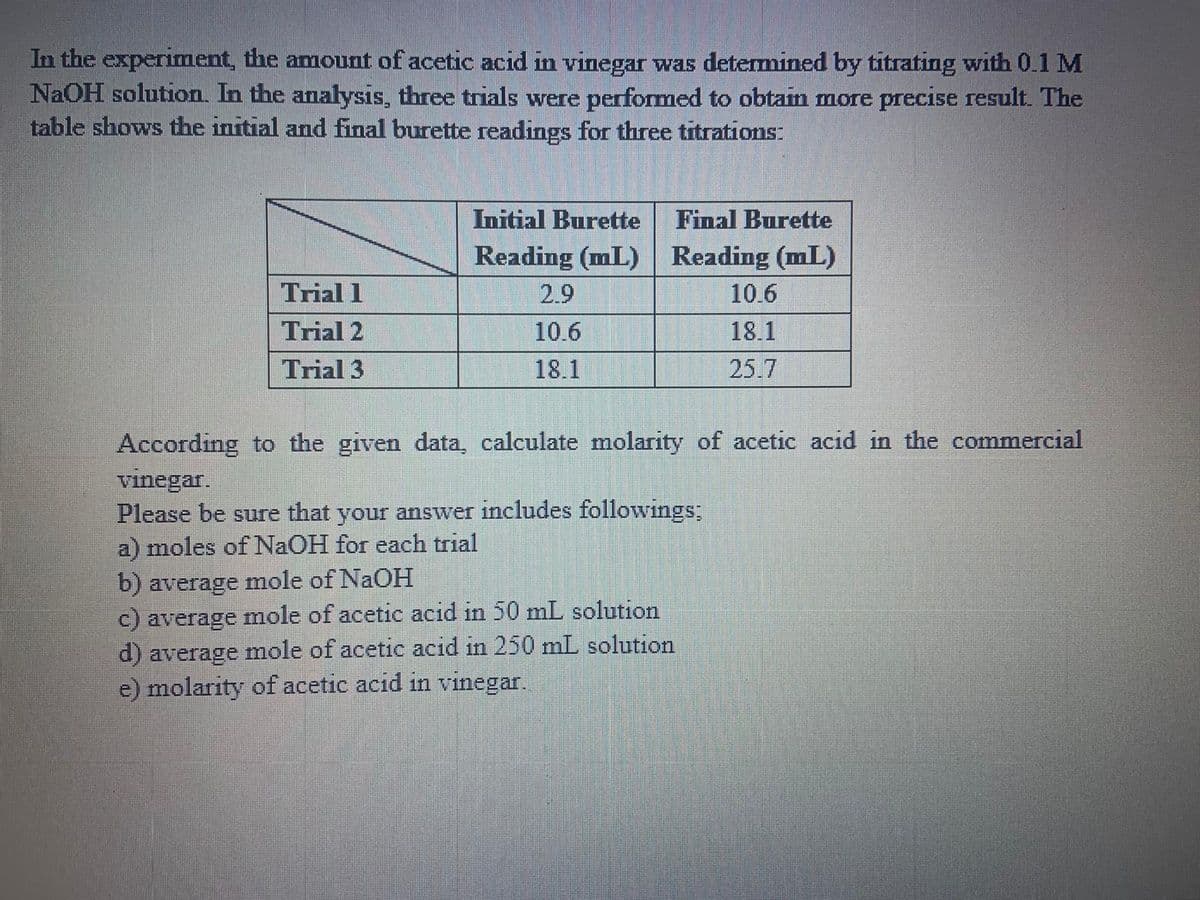
In the experiment, the amount of acetic acid in vinegar was determined by titrating with 0.1 M NaOH solution. In the analysis, three trials were performed to obtain more precise result. The table shows the initial and final burette readings for three titrations:
|
|
Initial Burette Reading (mL) |
Final Burette Reading (mL) |
|
Trial 1 |
2.9 |
10.6 |
|
Trial 2 |
10.6 |
18.1 |
|
Trial 3 |
18.1 |
25.7 |
According to the given data, calculate molarity of acetic acid in the commercial vinegar.
Please be sure that your answer includes followings;
a) moles of NaOH for each trial
b) average mole of NaOH
c) average mole of acetic acid in 50 mL solution
d) average mole of acetic acid in 250 mL solution
e) molarity of acetic acid in vinegar.
Step by step
Solved in 7 steps









