Mass spectral measurements were made on 10 replicate low concentration Ni-EDTA samples: 191.1, 150.8, 160.7, 175.7, 144.7, 214.1, 165.7, 170.3, 159.7, and 177.3 counts. Ten measurements of a blank had a mean measurement of 38.3 counts. A sample containing 1.00 µM Ni-EDTA gave a measurement of 1739 counts. Estimate the signal and concentration detection limits for Ni- EDTA. signal detection limit: 99.02 counts 0.0714 concentration detection limit: uM Incorrect
Mass spectral measurements were made on 10 replicate low concentration Ni-EDTA samples: 191.1, 150.8, 160.7, 175.7, 144.7, 214.1, 165.7, 170.3, 159.7, and 177.3 counts. Ten measurements of a blank had a mean measurement of 38.3 counts. A sample containing 1.00 µM Ni-EDTA gave a measurement of 1739 counts. Estimate the signal and concentration detection limits for Ni- EDTA. signal detection limit: 99.02 counts 0.0714 concentration detection limit: uM Incorrect
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter13: An Introduction To Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 13.14QAP
Related questions
Question
I need only hand written answer
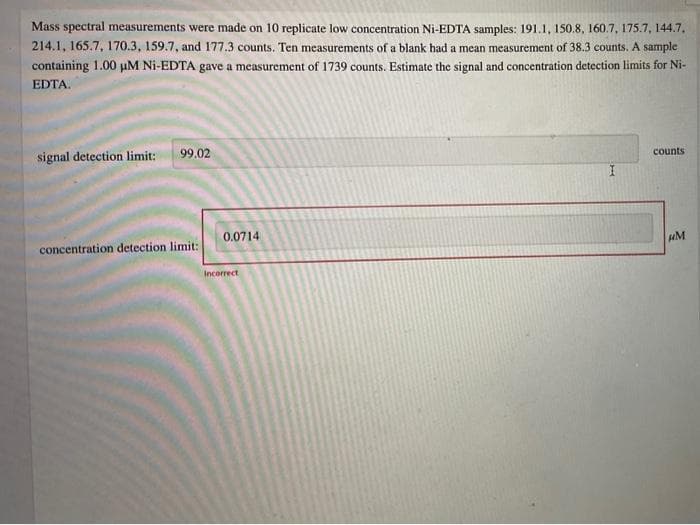
Transcribed Image Text:Mass spectral measurements were made on 10 replicate low concentration Ni-EDTA samples: 191.1, 150.8, 160.7, 175.7, 144.7,
214.1, 165.7, 170.3, 159.7, and 177.3 counts. Ten measurements of a blank had a mean measurement of 38.3 counts. A sample
containing 1.00 µM Ni-EDTA gave a measurement of 1739 counts. Estimate the signal and concentration detection limits for Ni-
EDTA.
99.02
counts
signal detection limit:
0.0714
uM
concentration detection limit:
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning