Q: A 100.0 mL sample containing Zn2+ was treated with 50.0 mL of 0.0500 M EDTA to complex all the Zn2+…
A: We have given that To calculate Concentration of Zn2+ =M1 = ? Volume of Zn2+ =V1 = 100ml Volume of…
Q: A 50.00 ml aliquot solution containing 0.524 g of magnesium sulfate (FW 120.37 in 0.500 L required…
A: Given that - Volume of magnesium sulfate = 50.00 mL Mass of magnesium sulfate in 0.500 L Solution…
Q: You are a chemist, you know that in the analysis for total hardness, formation of Ca-EDTA complex in…
A:
Q: Pt (II) and TI (III) are two metals that can be determined by UV spectrophotometry with…
A: Given: The molar absorptivity coefficient for Tl (III) at a max of 621 nm is 5.64×104 M-1 cm-1. The…
Q: A 25.0 mL aliquot of 0.0660 M EDTA was added to a 46.0 mL solution containing an unknown…
A: Total concentration of EDTA = 0.0660 M Volume of EDTA = 25 mL EDTA reacts with V3+ and Ga3+
Q: 50.0-mL sample containing Ni2+ was treated with 25.0 mL of 0.0500 M EDTA to complex all the Ni2+ and…
A:
Q: If Ksp (AgBr) = 4.0 x 10-¹3, and if, for the Ag+ + NH3 complex system (Ag+ + NH3 ⇒ [AgNH3]*;…
A: Solubility product of sparingly soluble salt is given as the product of concentration of dissolved…
Q: Solution of EDTA is particularly valuable as titrants because the reagent combines with metal ions…
A: The chemical equation for the reaction of the formation of the Fe(II)-EDTA and Fe(III)-EDTA and the…
Q: A 50.0-mL sample containing Ni21 was treated with 25.0 mL of 0.050 0 M EDTA to complex all the Ni21…
A: Given: 25.0 mL of 0.050 M EDTA First calculate the moles present in 0.050 M EDTA.
Q: A 100, 0mL containing Zn^ 2+ was treated with 50.0 of 0.0500 M EDTA complex all the Zn^ 2+ and leave…
A: We have given that 100mL containing Zn2+ was treated with 50.0ml of 0.0500 M EDTA complex excess…
Q: A 50.0 mL sample containing Cd²+ and Mn²+ was treated with 60.9 mL of 0.0700 M EDTA. Titration of…
A: Volume of the sample = 50.0 mL…
Q: Why is it necessary to buffer the solution to a pH between 10 and 12 during the complexometric…
A: In this question, the reason for the need of buffer the solution to a pH between 10 and 12 during…
Q: Is it posible to remove 99% of 1.0 μM CuY2- impurity (by reduction to solid Cu) from a 10.0 mM CoY2-…
A: We mainly have cobalt-EDTA solution (CoY2-) in which there is a little bit of copper impurity which…
Q: A 0.60004g sample of Ni/Cu condenser tubing was dissolved in acid and diluted to 100.0mL in a…
A: Mass of the sample present in 0.1 L = 0.60004 g Mass of the sample present in 0.025 L = 0.25 ×…
Q: A 25.00 mL aliquot of a solution containing Cu2+ and Fe3+ was titrated with 17.08 mL of 0.05095 M…
A: The titration is a method for the estimation of the concentration of ions. The titration that leads…
Q: A 25 ml solution of unkown containing Fe+3 and Ca+2 required 16.06 ml of 0.051 M EDTA for complete…
A: Back titration is a process where the unknown concentration of an analyte is determined by reacting…
Q: A 25.00-mL sample containing Fe31 was treated with 10.00 mL of 0.036 7 M EDTA to complex all the…
A: Given data,Volume of sample=25.0mL=0.025LTotal EDTA added=10.0mL=0.010LMolarity fo…
Q: Explain these question
A: As per our guidelines, we are allowed to answer the first three subparts for you. Kindly repost the…
Q: the dispensing of the EDTA solution is discontinued when the color turns to purple. Because of this…
A: The Complexometric titration is involves the formation of complex between ligand and metal ion…
Q: a) What is the equilibrium that influences the colour change of the Eriochrome Black T indicator?…
A: EDTA is ethylenediaminetetraacetic acid which is an indicator. It has 2 amino and 4 carboxyl groups.…
Q: You wish to measure the iron content of the well water on the new property you are about to buy. You…
A: Interpretation - To calculate the concentration of iron in the well water is to be explained.
Q: A 25.00 mL sample containing an unknown amount of Al3+ and Pb2+ required 19.13 mL of 0.04842 M EDTA…
A:
Q: In the analysis for total hardness, formation of Ca-EDTA complex in the presence of an…
A: We have to find the expression corresponding to KMg-EDTA.
Q: EDTA abstracts bismuth(III) from its thiourea complex: Bi1tu26 31 1 H2Y22 S BiY2 1 6tu 1 2H1 where…
A: The photometric titration curve involves the plot of the absorbance of a solution against the volume…
Q: Why do you think test tube D produces a pale color as compared to the control, test tube A when KCI…
A: The complexes in which d-block elements are surrounded by atoms, molecules and ions then it is…
Q: a 10 ml sample containing fe3+ and cu2+ was diluted to 100.0 mL solution. A 20 mL aliquot required…
A: Answer is explained below.
Q: In the analysis for total hardness, formation of Ca-EDTA complex in the presence of an…
A: We have to find equilibrium constant of a reaction based on given reactions.
Q: A cyanide solution with a volume of 13.87 mL was treated with 24.00 mL of Ni+ solution (containing…
A: Concentration of CN- can be determined from the amount of Ni2+ used in the titration of CN-.
Q: A 100.0 mL sample containing Zn2* was treated with 50.0 mL of 0.0500 M EDTA to complex all the Zn2+…
A: The given titration involves the following reactiion: H2Y2- + Zn2+…
Q: A 25.00-mL sample containing Fe3+ was treated with 10.00 mL of 0.036 7 M EDTA to complex all the…
A: In the question Following information given - Volume of Fe3+ Solution = 25 mL Volume of EDTA = 10 mL…
Q: chip sample) is analyzed using EDTA titration with EBT as indicator. The following data were…
A:
Q: Question 7: A 50.0 mL sample containing Fe3+ and Cu2+ required 35.20 mL of 0.05083 M EDTA for…
A: The given question is base on the quantitative analysis. This reaction involves EDTA titration. So,…
Q: Calculate pCd²+ at each of the given points in the titration of 60.00 mL of 0.0060 M Cd²+ with…
A:
Q: Fe^3+(aq) + SCN^-(aq) FESCN^2+(aq) Iron(II) thiocyanate is produced. This species can be determined…
A: Since the Fe3+ and SCN- reacts in 1:1, and the amount of SCN- is less than Fe3+, thus SCN- is the…
Q: Indirect EDTA determination of cesium. Cesium ion does not form a strong EDTA complex, but it can be…
A:
Q: A small amount of Mg-EDTA is often added in the titrimetric determination of water hardness. Why?…
A: EDTA a complexing agent designed to bind metal ions quantitatively, forming stable, water soluble…
Q: A 0.6006-g sample of Ni/Cu condenser tubing was dissolved in acid and diluted to 100 mL in a…
A:
Q: Why is EDTA complex formation less complete at lower pH?
A: Ethylenediamine tetraacetic acid (EDTA) is a polyprotic acid containing four carboxylic acid groups…
Q: A 25.0 mL aliquot of 0.0700 M EDTA was added to a 32.0 mL solution containing an unknown…
A: Interpretation - The original concentration of the V3+ solution is to be explained. Given…
Q: Poly (ethylene oxide), PEO (Tg = -60oC), is able to complex with cations such as Li+ and Na+, and…
A: Poly (ethylene oxide), PEO (Tg = -60oC), is able to complex with cations such as Li+ and Na+, and…
Q: A 15.0 mL sample of 0.0250 M Cu²+ buffered at pH 8.00 is titrated with 0.0100 M EDTA. Calculate…
A: Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: Example: Calculate the equilibrium concentrations of metal in aqueous and organic phases if 50 cm3…
A:
Q: ndirect EDTA determination of cesium. Cesium ion does not form a strong EDTA complex, but it can be…
A: Solution - According to the question - Given - mmol of Bi in reaction = 23ml * 0.07719M = 1.77 mmol…
Q: Molar absorptivities of Nickel and Cobalt with 8-quinilinol are εCo = 3529 and εNi = 3228 at 365 nm,…
A: Wavelength Co Ni Molar Absorptivity 365 nm 3529 3228 Molar Absorptivity 700 nm 428.9 0…
Q: You are asked to titrate a Mn3+ solution with EDTA at pH 9.00. The overall ionic strength of the…
A: Solution- Log k = 25.2k =1025.2 nowαEDTA4−=5.4 ×10−2×1025.2 at pH=9(a) k'(conditional formation…
Q: a) Sketch the molecular structure of EDTA. Indicate the ligands in your sketch.
A:
Q: 10. A 50.0ml solution containing Ni2+ and Zn2+ was treated with 25.0 ml 0.0452M EDTA to bind all the…
A: The titration in which the formation of colored complex is used to determine the endpoint, are known…
Q: You are a chemist, you know that in the analysis for total hardness, formation of Ca-EDTA complex in…
A: A question based on equilibrium concept, which is to be accomplished.
You are a chemist, you know that in the analysis for total hardness, formation of Ca-EDTA complex in the presence of magnesium-calmagite indicator or Mg-Ind complex involves the following process:
Based on the
a. [Mg-EDTA] / {[Mg2+] [EDTA2-]}
b. {[Mg-EDTA] [Ind2-]} / {Mg-Ind] [EDTA2-]}
c. [Mg2+] [EDTA2-]
d. {[Mg2+] [EDTA2-]} / [Mg-EDTA] {[Mg-EDTA]
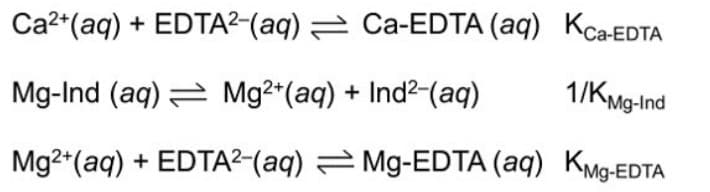
Step by step
Solved in 2 steps

- Aecrt5FT-IR technique can be utilized for the analysis of unknown analytes by matching it with__________. Both choices are correct Commercial library/database Reference standardPreparing a standard curve. 1. determine the absorbance max of [FeSCN]2+ it is 448.1 2. determine the concentration of FeSCN2+ in the stock solution. M1V1=M2V2 stock solution: ~0.200 M iron (iii) nitrate in 1M nitric acid intial volume: 0.3mL final volume: 10.3mL volume: 10mL ~0.002M potassium thiocyanate initial volume: 0.3mL final volume: 10.3mL volume: 10mL not sure if this is needed stock solutions: ~0.200M iron (iii) nitrate in 1M nitric acid: 0.207M ~0.002M iron (iii) nitrate in 1M nitric acid: 0.00209M ~0.002M potassium thiocyanate: 0.00193M
- Calculate the Ksp for Ni(OH)2 Ni(OH)2(s) + 2 e- ⟶ Ni(s) + 2OH-(aq); Eo = -0.720 VNi2+(aq) + 2 e- ⟶ Ni(s); Eo = -0.250 V 7.56x1015 1.15x10-8 1.70x10-33 1.32x10-16(9.29 × 105/8)-20.81 = with correct sig figsYou obtained the following raw data when setting up a Bradford standard curve: BSA (mg/ml) Absorbancy 595nm 0 0.225 1 0.310 2 0.420 3 0.510 4 0.610 5 0.720 6 0.810 7 0.915 8 0.950 9 0.980 10 0.990 After blanking against a bradford-dH2O sample, the protein concentration of an unknown sample was determined using the same method and an absorbancy of 0.523 was obtained. Set up a standard curve, excluding outliers (experimental and statistical) and determine the protein concentration in the unknown sample in mg / ml (up to 3 significant figures).
- You determine the acetic acid (HOAc) content of vinegar by titrating with a sodium hydroxide standard solution to a phenolphthalein (an indicator) end point. An approximately 5-mL sample of vinegar is weighed on an analytical balance in a weighing boat and this is found to be 5.0268 g. The standard deviation in making a single weighing is 0.2 mg. The sodium hydroxide must be accurately standardised (this means its concentration must be accurately determined) by titrating known mass of high-purity potassium hydrogen phthalate, and three such titrations give molar concentrations of 0.1167, 0.1163 and 0.1164 mol L-1. A volume of 36.78 mL of sodium hydroxide is used to titrate each sample. The standard deviation of the burette used is 0.02 mL. Calculate the percentage of acetic acid in the vinegar and its standard deviation.You are trying to come up with a drug to inhibit the activity of an enzyme thought to have a role in liver disease. In the laboratory the enzyme was shown to have a Km of 1.0 x 10-6 M and Vmax of 0.1 micromoles/min.mg measured at room temperature. You developed an uncompetitive inhibitor. In the presence of 5.0 x 10-5 M inhibitor, the apparent Vmax was determined to be 0.02 micromoles/min.mg. What is the Ki of the inhibitor?Felodipine calcium channel blocker standard (0.251mg/ml) and felodipine sample (0.245mg/ml) solutions were prepared and injected to the HPLC. The peak area of felodipine standard is 275428 and the sample is 272982. The potency (purity) of felodipine standard is 98.9%. What is the assay percentage of felodipine? a. 102.23 b. 98.71 c. 101.07 d. 100.42
- As part of an analytical chemistry laboratory course, a student measured the Ca2+ content in two water samples, city-supplied drinking water and well-supplied drinking water, using two different analytical methods, flame atomic absorption spectrometry (FAAS) and EDTA complexometric titration. The results of this experiment are given in the table as the mean Ca2+concentration (?¯) and standard deviation (?) in parts per million (ppm). Each sample was measured five times (n=5) by each method. Method City-Supplied Drinking Water (?¯±?x¯±s) Well-Supplied Drinking Water (?¯±?x¯±s) FAAS 57.57±0.68 ppm 64.77±0.70 ppm EDTA titration 58.32±0.96 ppm 65.62±0.97 ppm Method Comparison: For each drinking water sample (city and well), compare the Ca2+ content measured by FAAS and EDTA titration. Calculate the ? value for each sample. Do the methods produce statistically different results at the 95% confidence level when measuring the Ca2+content of the city-supplied drinking water? Do the…Anionic surfactants in wastewater are measured spectrophotometrically as methylene blue active substances (MBAS). A volume of a water sample is first reacted with methylene blue, a cationic dye, in a basic buffered aqueous solution. The resulting solution is agitated after the addition of chloroform. The organic layer is then extracted, and the absorbance is read at 655 nm. A standard calibration curve is produced by plotting absorbance vs. concentration for the concentration range between 0 ppm to 2.50 ppm. The line had the following equation: y = 0.286x + 0.009 a. The limit of surfactant content in Philippine class B water is 300 ppb. If a 5 mL aliquot of a 50 mL water sample had an absorbance of 0.084 after MBAS analysis, is the sampled water body suitable for bathing? Support your answeranalyte concentration(C)(mg/ml) injection volume (ul) elution time (time) peak DAD signal(mAU) caffeine 1 1 4.67 302.85 aspartame 5 1 7.53 15.83 benzoic acid 1 1 8.14 89.98 saccharin 1 1 1.91 84.86 mixture(add everything above with 1:1:1:1 ratio) 1 4.47 69.58 How to get the concentration of the mixture in this case?



