Molar concentration of KMNO 0.02000 M 0.2189 3 7mL 0.3 mL 36.7mL Branhish Weight of complex iron salt used 0.2009 2em 2159 23.6 Final buret reading 1.9 mL 21.7m2 Initial buret reading Volume of KMno, used 25ML Pink-PUYde Con (mol) Goranmanm RESULTS Sample 1 Sample 2 Sample 3 Moles Mno. used in titration Moles C,0," in sample Moles C,0. per gram of salt Average moles C,O." per gram of salt Write a balanced chemical equation for the reaction of permanganate with oxalate. 5C,0, Ca9)+ 2MaO,"Caa) H&H°Car)10 Co.G)+2Mn²° Caq) +8H220C1) Sample calculations
Molar concentration of KMNO 0.02000 M 0.2189 3 7mL 0.3 mL 36.7mL Branhish Weight of complex iron salt used 0.2009 2em 2159 23.6 Final buret reading 1.9 mL 21.7m2 Initial buret reading Volume of KMno, used 25ML Pink-PUYde Con (mol) Goranmanm RESULTS Sample 1 Sample 2 Sample 3 Moles Mno. used in titration Moles C,0," in sample Moles C,0. per gram of salt Average moles C,O." per gram of salt Write a balanced chemical equation for the reaction of permanganate with oxalate. 5C,0, Ca9)+ 2MaO,"Caa) H&H°Car)10 Co.G)+2Mn²° Caq) +8H220C1) Sample calculations
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.14QAP
Related questions
Question
100%
11
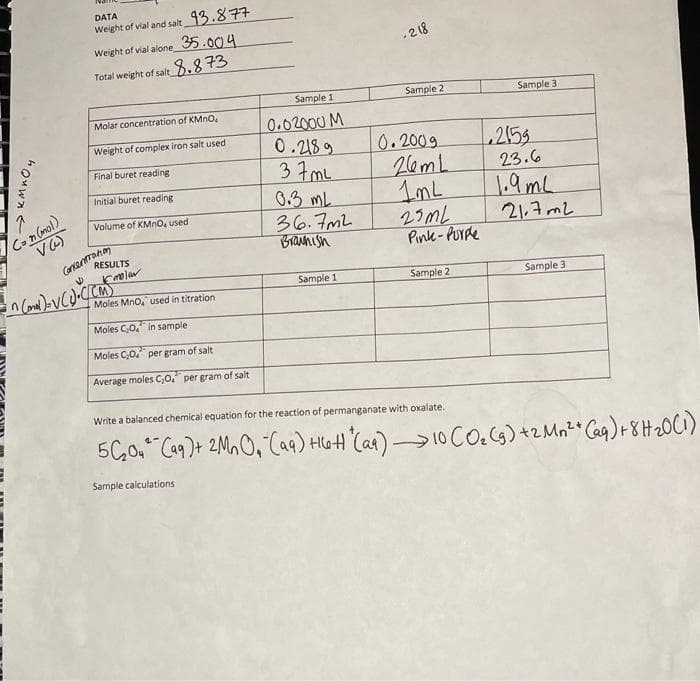
Transcribed Image Text:DATA
Weight of vial and salt 13.877
Weight of vial alone 5.004
•2 18
Total weight of salt 8.8 73
Sample 1
Sample 2
Sample 3
Molar concentration of KMNO.
0.02000 M
0.218 9
37 mL
0.3 mL
36.7mL
Brannish
Weight of complex iron salt used
0.2009
2153
23.6
1.9mL
21.7m2
Final buret reading
26mL
Initial buret reading
Volume of KMNO, used
Ca n (mol)
Granrahm
RESULTS
27ML
Pink-PUrde
Sample 1
Sample 2
Sample 3
Moles Mno, used in titration
Moles C,0, in sample
Moles C;0. per gram of salt
Average moles C,0." per gram of salt
Write a balanced chemical equation for the reaction of permanganate with oxalate.
5C,0, Ca9)+ 2Ma O,"Caq) HGH"(Car) 10 Co.cs) +2 Mn²*Caq)+ 8H20C1)
Sample calculations
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

