Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter22: Organic Chemistry
Section: Chapter Questions
Problem 28QAP: When the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction...
Related questions
Question
A reaction for converting
Baeyer–Villiger reaction,
is used in the manufacture of plastics and pharmaceuticals.
3-Chloroperbenzoic acid is shock-sensitive, however,
and prone to explode. Also, 3-chlorobenzoic acid is
a waste product. An alternative process being developed
uses hydrogen peroxide and a catalyst consisting of tin
deposited within a solid support. The catalyst is readily
recovered from the reaction mixture. (a) What would
you expect to be the other product of oxidation of the
ketone to lactone by hydrogen peroxide? (b) What principles
of green chemistry are addressed by use of the proposed
process?
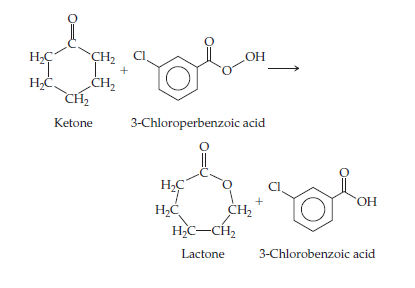
Transcribed Image Text:НС
ҫH2
Он
НС
CH2
CH2
Ketone
3-Chloroperbenzoic acid
НС
ОН
НС,
CH2
H,C-CH2
Lactone
3-Chlorobenzoic acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning