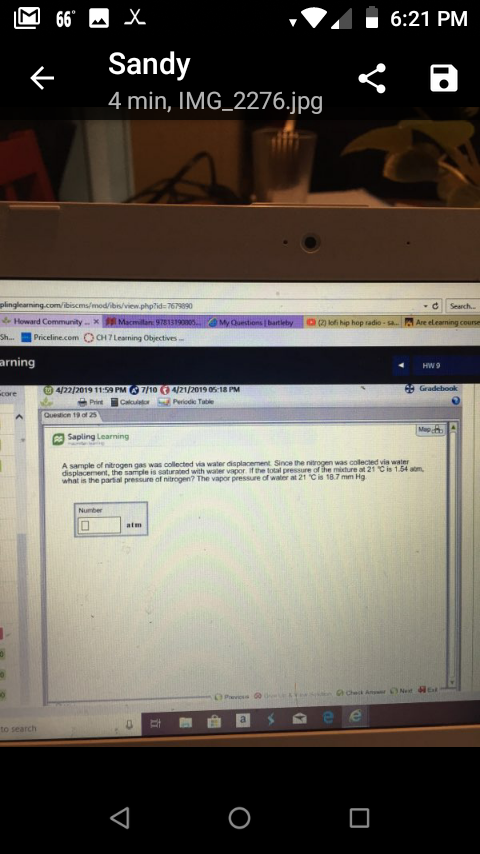
p 6:21 PM Sandy 4 min, IMG_2276.jpg Search 7679890 & Howard CommunityX Sha, ■ Priceline.com OCH 7 Lewning Otyectves arning (2) lofi hip hop tadio-sa. Are eLearningcourse HW 4/22/2019 11:59 PM 7/10 421/2019 05 18 PM Saping Learning was colected vi A sample of nitrogen gas was collected via water displacement Since the displacement, the sampl" is saturated with water vagor if the total pressure of he notre at 21 ℃ is 1 54 am what is the partal pressure of nitrogen? The vapor pressure the toal pres 1 Cis 18.7 mm Hg or water at 21 C is 18.7 mm Hg to search
p 6:21 PM Sandy 4 min, IMG_2276.jpg Search 7679890 & Howard CommunityX Sha, ■ Priceline.com OCH 7 Lewning Otyectves arning (2) lofi hip hop tadio-sa. Are eLearningcourse HW 4/22/2019 11:59 PM 7/10 421/2019 05 18 PM Saping Learning was colected vi A sample of nitrogen gas was collected via water displacement Since the displacement, the sampl" is saturated with water vagor if the total pressure of he notre at 21 ℃ is 1 54 am what is the partal pressure of nitrogen? The vapor pressure the toal pres 1 Cis 18.7 mm Hg or water at 21 C is 18.7 mm Hg to search
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 93QRT
Related questions
Question
100%
Transcribed Image Text:p
6:21 PM
Sandy
4 min, IMG_2276.jpg
Search
7679890
& Howard CommunityX
Sha, ■ Priceline.com OCH 7 Lewning Otyectves
arning
(2) lofi hip hop tadio-sa.
Are eLearningcourse
HW
4/22/2019 11:59 PM
7/10 421/2019 05 18 PM
Saping Learning
was colected vi
A sample of nitrogen gas was collected via water displacement Since the
displacement, the sampl" is saturated with water vagor if the total pressure of he notre at 21 ℃ is 1 54 am
what is the partal pressure of nitrogen? The vapor pressure
the toal pres 1 Cis 18.7 mm Hg
or water at 21 C is 18.7 mm Hg
to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning