Part D Find the number of moles of water that can be formed if you have 178 mol of hydrogen gas and 84 mol of oxygen gas. Express your answer with the appropriate units. , View Available Hint(s) 123+ Value Units Submit
Part D Find the number of moles of water that can be formed if you have 178 mol of hydrogen gas and 84 mol of oxygen gas. Express your answer with the appropriate units. , View Available Hint(s) 123+ Value Units Submit
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 35A
Related questions
Question
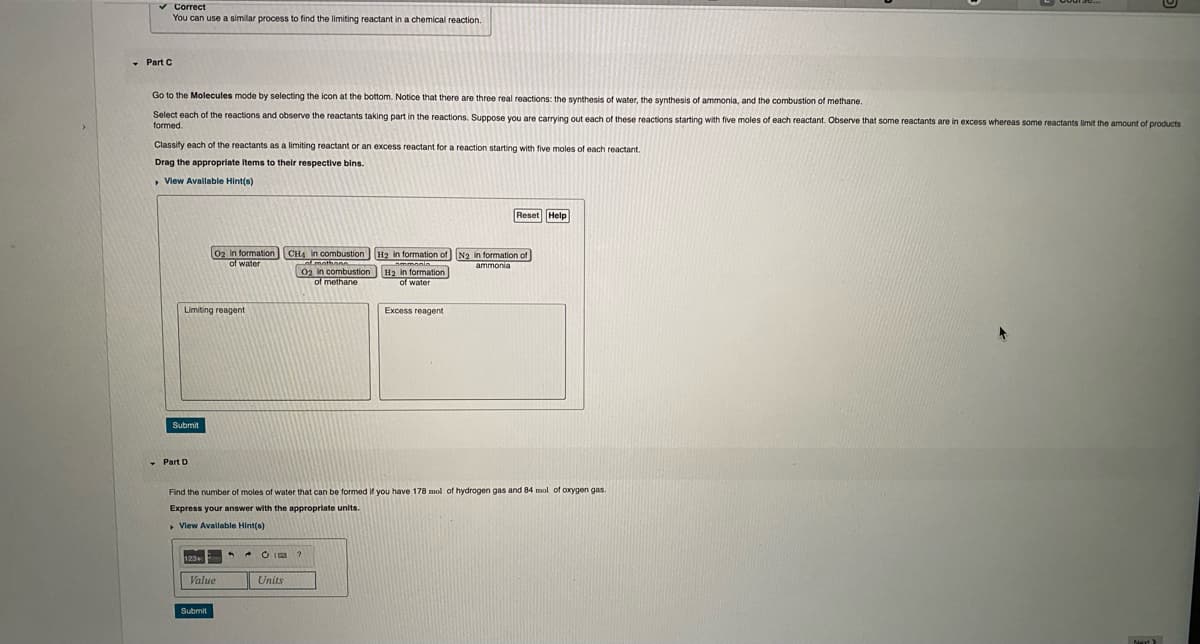
Transcribed Image Text:v Correct
You can use a similar process to find the limiting reactant in a chemical reaction.
- Part C
Go to the Molecules mode by selecting the icon at the bottom. Notice that there are three real reactions: the synthesis of water, the synthesis of ammonia, and the combustion of methane.
Select each of the reactions and observe the reactants taking part in the reactions. Suppose you are carrying out each of these reactions starting with five moles of each reactant. Observe that some reactants are in excess whereas some reactants limit the amount of products
formed.
Classity each of the reactants as a limiting reactant or an excess reactant for a reaction starting with five moles of each reactant.
Drag the appropriate items to their respective bins.
• View Avallable Hint(s)
Reset
02 in formation
of water
CH4 in combustion H2 in formation of N2 in formation of
02 in combustion H2 in formation
of methane
Limiting reagent
Excess reagent
Submit
Part D
Find the number of moles of water that can be formed if you have 178 mol of hydrogen gas and 84 mol of oxygen gas
Express your answer with the appropriate units.
, Vlew Available Hint(s)
123
* * O IE ?
Value
Units
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co