Please demonstrate how to do question 1 parts a, b, and c. You do not need to do them all, simply walk me through on how to do the calculations.
Please demonstrate how to do question 1 parts a, b, and c. You do not need to do them all, simply walk me through on how to do the calculations.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter5: Gases
Section: Chapter Questions
Problem 101E: At elevated temperatures, sodium chlorate decomposes to produce sodium chloride and oxygen gas. A...
Related questions
Question
Please demonstrate how to do question 1 parts a, b, and c.
You do not need to do them all, simply walk me through on how to do the calculations.
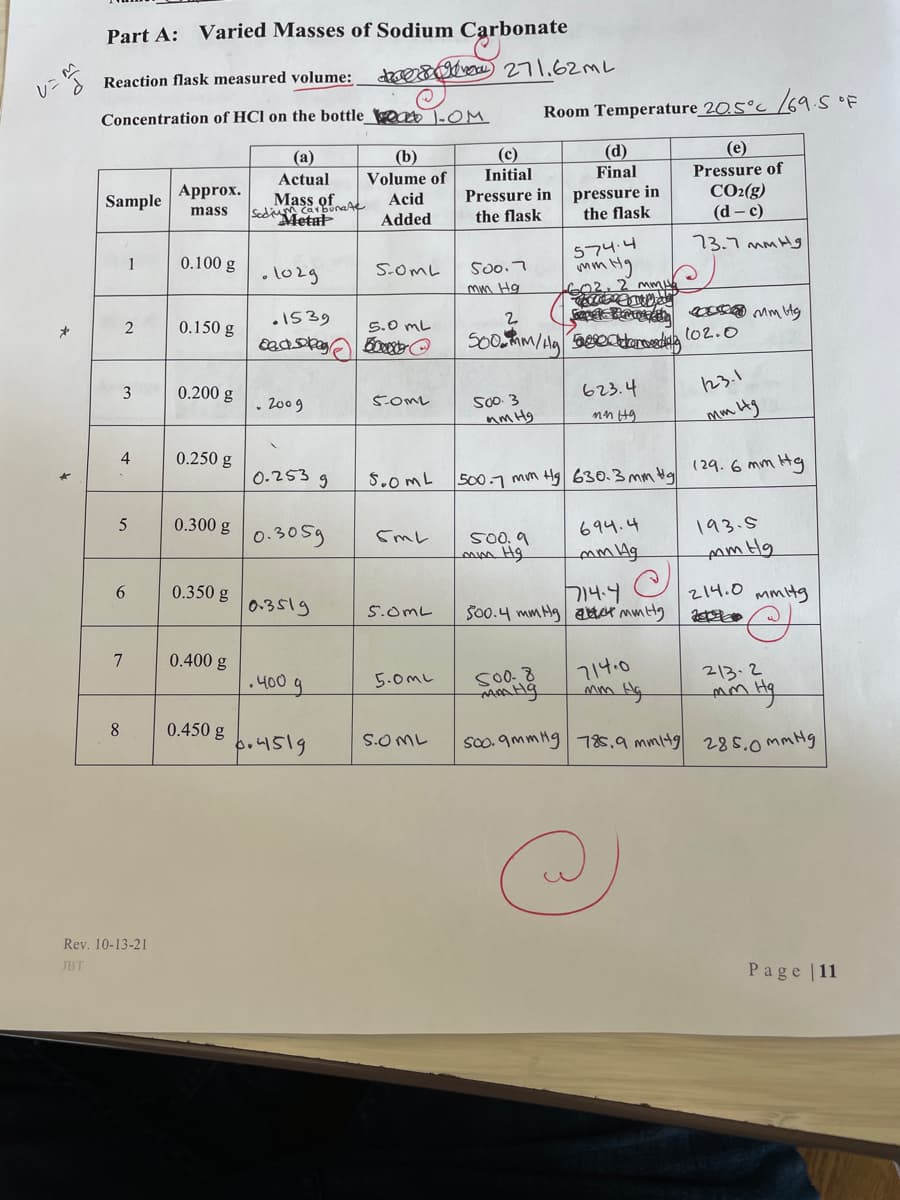
Transcribed Image Text:Part A: Varied Masses of Sodium Carbonate
* Reaction flask measured volume:
dsevo 271.62ML
レニ
Concentration of HCl on the bottle_ o 1-OM
Room Temperature_20.5°c /69.S OF
(a)
(e)
(d)
Final
(b)
(c)
Actual
Volume of
Initial
Pressure of
Approx.
Sample
Mass of
Sedium car bunaA
Metal
pressure in
the flask
CO2(g)
(d – c)
Acid
Pressure in
mass
Added
the flask
73.7 mm Hg
574.4
mm'
1
0.100 g
•lo2g
S.OmL
500.7
_mm Hg
2 mm
.1539
0.150 g
5.0 mL
3
0.200 g
623.4
23.1
. 2009
SOML
500.3
nm Hg
4
0.250 g
0-253 g
S.0mL
S0o.7 mm Hg 630.3 mm Ha (29.6 mm Hg
0.300 g
0.305g
694.4
193.5
500, 9
mm Hg
mm Hg
6
0.350 g
0.3519
714.4
214.0 mmitg
5.0mL
7
0.400 g
714.0
mm Hg
.400
5.0mL
213.2
8.
0.450 g
bo4519
soo. 9mmHg 78s,9 mmi4g 285.ommHg
S.OML
Rev. 10-13-21
JBT
Page 11
2.

Transcribed Image Text:Post-laboratory Assignment
(page 1 of 4)
Excel Spreadsheet:
Part A:
Room
Use Excel to generate a spreadsheet with the following information.
(d)
rature
Column
ressre
Enter the measured masses of sodium carbonate from data page #1
Theoretical Moles CO2(g) – Using stoichiometry, calculate the theoretical number of
moles of CO2 that can be produced from each reactant entry.
Theoretical Pressure CO2(g) - Using the ideal gas law, measured volume, and
temperature, calculate the theoretical pressure of CO2(g) that can be produced from the
theoretical moles of CO2(g) in column B.
A
В
C
D
Actual measured pressures of carbon dioxide gas produced from data page #1
On your Excel spreadsheet, circle the mass of sodium carbonate in column A for when the
limiting reactant changes. Compare the values in column B to the answer for #1b below.
All sample calculations associated with the spreadsheet are MUST be completed in Q#1 of the
Post-Lab to receive credit for the spreadsheet.
1. Sample calculations using the quantities measured in the lab and the ideal gas law.
Part A
a. Calculate the theoretical number of moles of carbon dioxide gas produced using your
measured mass of sodium carbonate for the 0.100 grams of Na2CO3. (Sample calculation for
column B)
b. Calculate the theoretical mole quantity of carbon dioxide gas produced from 5.0 mL of
1.0 M HCl aqueous solution.
c. Calculate the theoretical pressure of carbon dioxide gas produced in the flask using the
reaction conditions and the quantities measured in the lab for the 0.100 g Na2CO3 trial.
(hint: consider your answers from a & b when doing this calculation.)
ev. 10-13-21
BT
Page |13
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

