Qualitative Analysis of Proteins 1. Fill out the table below by providing the necessary information indicated per column. 2. As you have virtually conducted these qualitative tests for proteins, what do you think could be the essential practical or real-life applications of these tests?
Qualitative Analysis of Proteins 1. Fill out the table below by providing the necessary information indicated per column. 2. As you have virtually conducted these qualitative tests for proteins, what do you think could be the essential practical or real-life applications of these tests?
Chapter8: Forms Of Drugs And How They Act
Section: Chapter Questions
Problem 30RQ
Related questions
Question
100%
Qualitative Analysis of Proteins
1. Fill out the table below by providing the necessary information indicated per column.
2. As you have virtually conducted these qualitative tests for proteins, what do you think could be the essential practical or real-life applications of these tests?
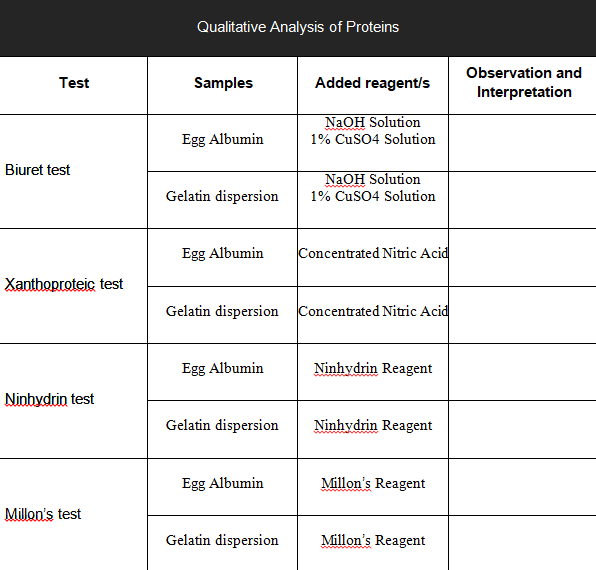
Transcribed Image Text:Qualitative Analysis of Proteins
Observation and
Test
Samples
Added reagent/s
Interpretation
NaOH Solution
1% CuSO4 Solution
Egg Albumin
Biuret test
NaOH Solution
1% CuSO4 Solution
Gelatin dispersion
Egg Albumin
Concentrated Nitric Acid
Xanthoproteic test
Gelatin dispersion Concentrated Nitric Acid
Egg Albumin
Ninhydrin Reagent
Ninbydrin test
Gelatin dispersion
Ninhydrin Reagent
Egg Albumin
Millon's Reagent
Millon's test
Gelatin dispersion
Millon's Reagent
Transcribed Image Text:Qualitative Analysis of Proteins
Qualitative Analysis of Proteins
HELP
HELP
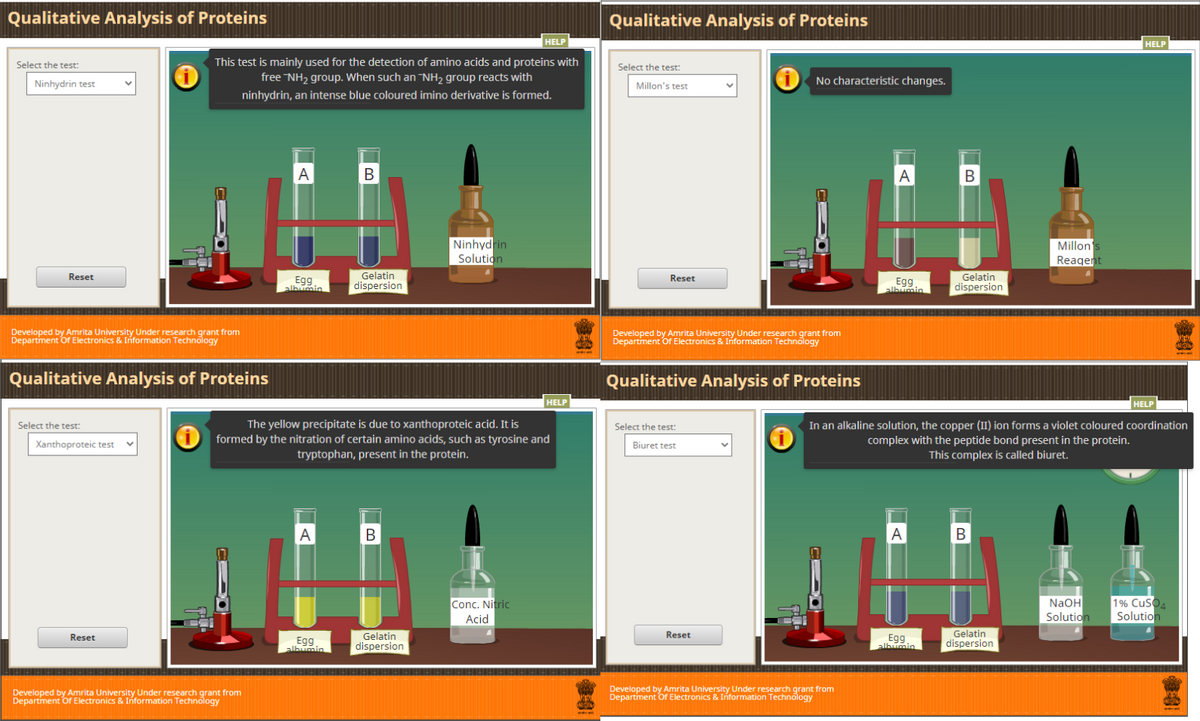
This test is mainly used for the detection of amino acids and proteins with
free NH2 group. When such an NH2 group reacts with
Select the test:
Select the test:
Ninhydrin test
No characteristic changes.
Millon's test
ninhydrin, an intense blue coloured imino derivative is formed.
A
В
A
В
Ninhydrin
Solution
Millon
Reagent
Gelatin
dispersion
Reset
Gelatin
dispersion
Reset
Egg.
albumin
Egg
albumin
Developed by Amrita University Under research grant from
Department Of Electronics & Information Technology
Developed by Amrita University Under research grant from
Department Of Electronics & Information Technology
Qualitative Analysis of Proteins
Qualitative Analysis of Proteins
HELP
HELP
The yellow precipitate is due to xanthoproteic acid. It is
formed by the nitration of certain amino acids, such as tyrosine and
tryptophan, present in the protein.
In an alkaline solution, the copper (II) ion forms a violet coloured coordination
complex with the peptide bond present in the protein.
This complex is called biuret.
Select the test:
Select the test:
Xanthoproteic test
Biuret test
A
В
A
В
NaOH
Solution
1% CusO4
Solution
Conc. Nitric
Acid
Gelatin
dispersion
Reset
Gelatin
dispersion
Reset
Egg
albumin
Egg.
albumin
Developed by Amrita University Under research grant from
Department Of Electronics & Ińformation Technology
Developed by Amrita University Under research grant from
Department Of Electronics & Information Technőlogy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

