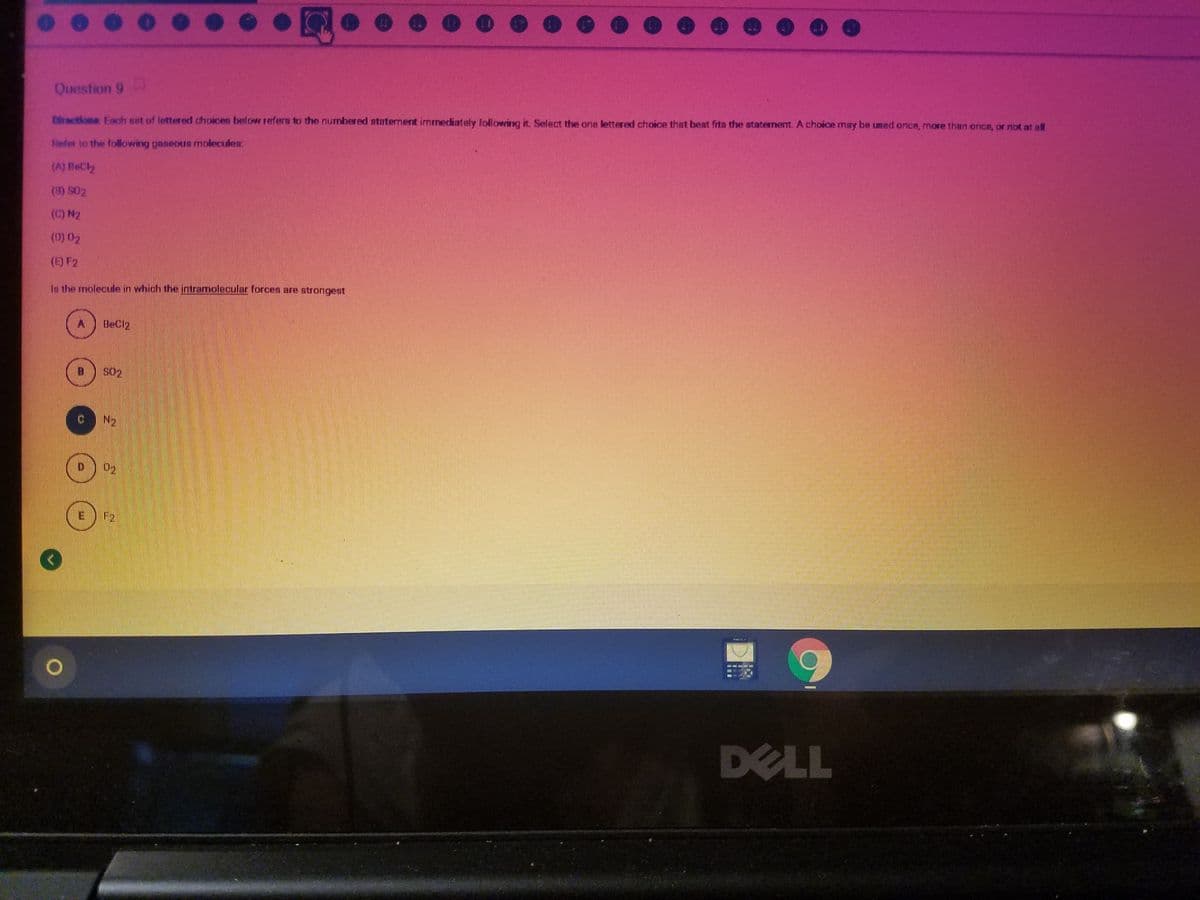
Question 9 Diractiona Fach set of lettered choices beloH refers to the numbered staternent immediately following it. Select the one lettered choice that best fita the statement.A choice may be umed once, more than once, or not at all Fefer to the following aneous molecuden (A) BeC (3) S02 (C) N2 (0) 02 (E) F2 Is the molecule in which the intramiolecular forcen are strongest BeCl2 S02 N2 02 F2
Question 9 Diractiona Fach set of lettered choices beloH refers to the numbered staternent immediately following it. Select the one lettered choice that best fita the statement.A choice may be umed once, more than once, or not at all Fefer to the following aneous molecuden (A) BeC (3) S02 (C) N2 (0) 02 (E) F2 Is the molecule in which the intramiolecular forcen are strongest BeCl2 S02 N2 02 F2
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter14: Rotational And Vibrational Spectroscopy
Section: Chapter Questions
Problem 14.96E
Related questions
Question
Can you solve the question in the picture shown?
Transcribed Image Text:...
...o 0.
Question 9
Diractiona Each set of lettered choices below refers to the numbered atatement immediately Tollowing it. Select the one lettered choice that best fita the ataterment. A choice may be umed once, more than once, or not at al
Refer to the following gaseous molecules:
(A) BeCl2
(8) S02
(0) N2
(E) F2
Is the molecule in which the intramolecular forces are sBtrongest
BeCl2
S02
N2
E
F2
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning