Formal Charges
Formal charges have an important role in organic chemistry since this concept helps us to know whether an atom in a molecule is neutral/bears a positive or negative charge. Even if some molecules are neutral, the atoms within that molecule need not be neutral atoms.
Polarity Of Water
In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. Polarity in any molecule occurs due to the differences in the electronegativities of the bonded atoms. Water, as we all know has two hydrogen atoms bonded to an oxygen atom. As oxygen is more electronegative than hydrogen thus, there exists polarity in the bonds which is why water is known as a polar solvent.
Valence Bond Theory Vbt
Valence bond theory (VBT) in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. It gives a quantum mechanical approach to the formation of covalent bonds with the help of wavefunctions using attractive and repulsive energies when two atoms are brought from infinity to their internuclear distance.
(a) Which one of the statements concerning valence bond (VB) and molecular orbital (MO) bond theories is correct?
A. In VB theory, bonding electrons are delocalized over the molecule.
B. MO theory accurately describes bonding in O2 and NO, VB theory does not.
C. MO theory predicts that electrons are localized between pairs of atoms.
D. MO theory is used to accurately predict the colors of compounds.
E. VB theory can describe molecular bonding in excited states.
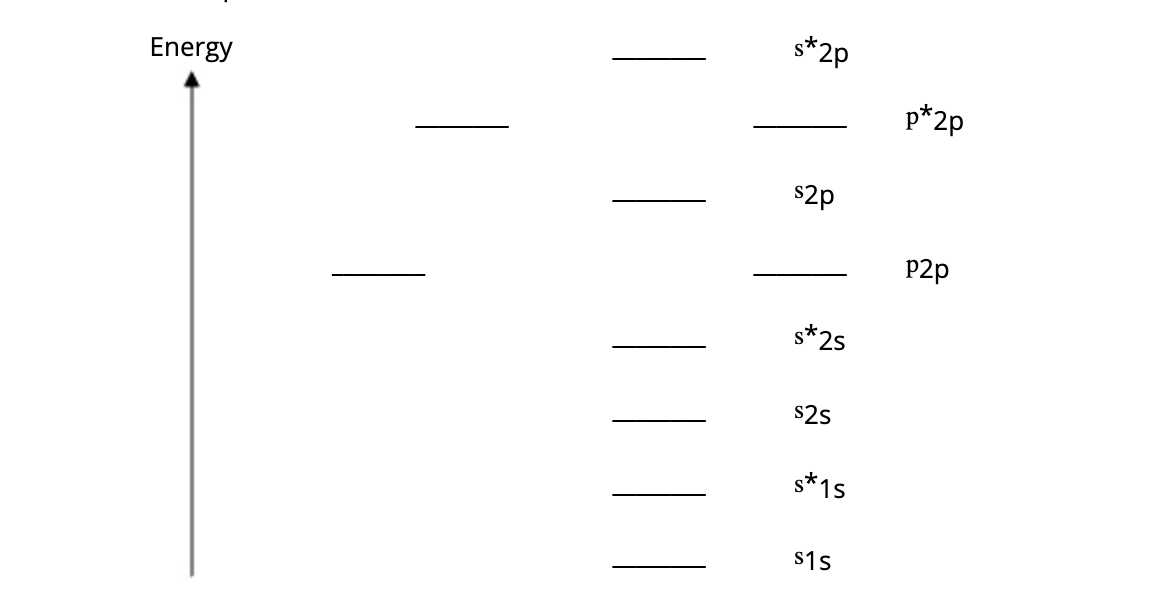
(b) Refer to Diagram 9-1. Use molecular orbital theory to predict which species is paramagnetic.
A. Li2
B. H2
C. F2
D. O2
E. N2
(c) Which combination of atoms is most likely to produce a compound with covalent bonds?
A. Al and O
B. Pb and F
C. K and I
D. S and Br
E. Na and Cl
(d) The central atom in the triiodide ion, I 3 –, is surrounded by
A. one single bond, one double bond, and one lone pair of electrons.
B. two single bonds and three lone pairs of electrons.
C. two single bonds and no lone pairs of electrons.
D. two single bonds and two lone pairs of electrons.
E. two double bonds and one lone pair of electrons.
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images






