Some chemical compounds are listed in the first column of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are written in the second column of the table. Use the checkboxes to classify each compound. compound КОН Nacio, GIGNI important species present when dissolved in water K.OH.HO NH, ĐỊNH HO Na, CIO, 1,0 сиин.он, сцин. но type of compound (check all that apply) lonic molecular strong weak strong weak acid acid base base 0 0 DO O 0 0 0 O O 0 0 0 0 0 O O 000 X 0 5 OC E
Some chemical compounds are listed in the first column of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are written in the second column of the table. Use the checkboxes to classify each compound. compound КОН Nacio, GIGNI important species present when dissolved in water K.OH.HO NH, ĐỊNH HO Na, CIO, 1,0 сиин.он, сцин. но type of compound (check all that apply) lonic molecular strong weak strong weak acid acid base base 0 0 DO O 0 0 0 O O 0 0 0 0 0 O O 000 X 0 5 OC E
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter7: Sollutions And Colloids
Section: Chapter Questions
Problem 7.5E: Use the term soluble, insoluble, or immiscible to describe the behavior of the following pairs of...
Related questions
Question
Give typed explanation of all subparts not a single word hand written otherwise leave it
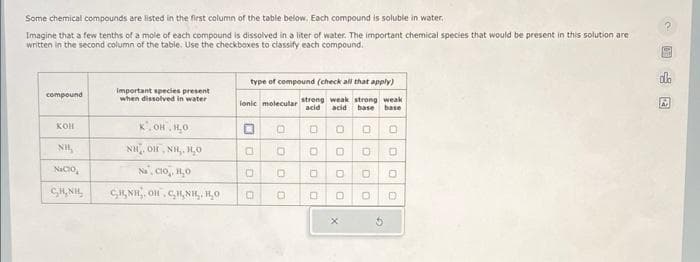
Transcribed Image Text:Some chemical compounds are listed in the first column of the table below. Each compound is soluble in water.
Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are
written in the second column of the table. Use the checkboxes to classify each compound.
compound
KOH
NH,
Nacio,
SH NH
Important species present
when dissolved in water
K.OH.H₂O
NH, OH NH, HO
Na, Cio. H₂O
CINE. OH CHNIL, RO
type of compound (check all that apply)
ionic molecular strong weak strong weak
acid acid base base
O
0
O
D
0
0 0
OO
0
0
10
0
0
0000
0
0
000
X
0
5
B
圆中国
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning