SPECTROPHOTOME TRY The DPD colorimetric method is used to determine the amount of residual chlorine in the sample. In this method, the free chlorine in the sample oxidizes the colorless amine N,N- diethyl-p-phenylenediamine to a colored compound that absorbs strongly at 520 nm. The analysis of a set of calibration standards gave the following results: ppm Cl2 0.00 0.50 1.00 1.50 2.00 2.50 3.00 Absorbance 0.0031 0.1232 0.3241 0.5438 0.7642 0.9551 1.1565 A 10.00 mL water sample from a public water supply was diluted to 250 mL and analyzed for free chlorine residual, giving an absorbance of 0.3210. A. Find the equation of the line. B. What is the molar absorptivity of Cl2 (FW = 70.9) at 250 nm? C. Calculate the free chlorine residual of the sample as ppm Cl2. D. Calculate ppm BaCl2 (FW = 208.2)?
SPECTROPHOTOME TRY The DPD colorimetric method is used to determine the amount of residual chlorine in the sample. In this method, the free chlorine in the sample oxidizes the colorless amine N,N- diethyl-p-phenylenediamine to a colored compound that absorbs strongly at 520 nm. The analysis of a set of calibration standards gave the following results: ppm Cl2 0.00 0.50 1.00 1.50 2.00 2.50 3.00 Absorbance 0.0031 0.1232 0.3241 0.5438 0.7642 0.9551 1.1565 A 10.00 mL water sample from a public water supply was diluted to 250 mL and analyzed for free chlorine residual, giving an absorbance of 0.3210. A. Find the equation of the line. B. What is the molar absorptivity of Cl2 (FW = 70.9) at 250 nm? C. Calculate the free chlorine residual of the sample as ppm Cl2. D. Calculate ppm BaCl2 (FW = 208.2)?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter1: Introduction
Section: Chapter Questions
Problem 1.11QAP
Related questions
Question
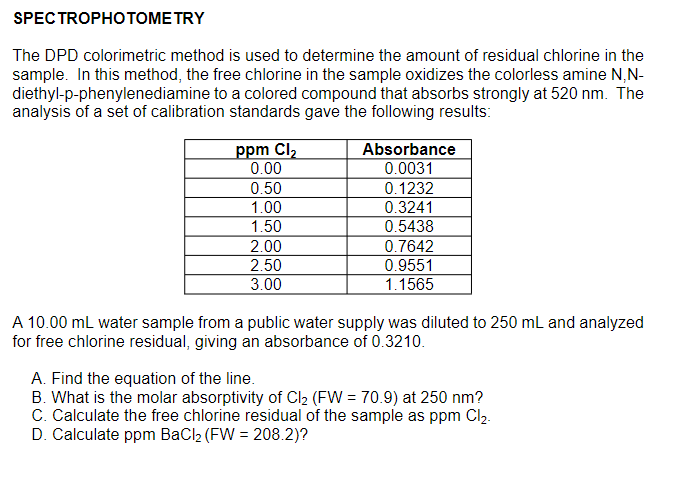
Transcribed Image Text:SPECTROPHOTОМЕ TRY
The DPD colorimetric method is used to determine the amount of residual chlorine in the
sample. In this method, the free chlorine in the sample oxidizes the colorless amine N,N-
diethyl-p-phenylenediamine to a colored compound that absorbs strongly at 520 nm. The
analysis of a set of calibration standards gave the following results:
Absorbance
0.0031
0.1232
0.3241
ppm Cl2
0.00
0.50
1.00
1.50
0.5438
2.00
0.7642
2.50
0.9551
3.00
1.1565
A 10.00 mL water sample from a public water supply was diluted to 250 mL and analyzed
for free chlorine residual, giving an absorbance of 0.3210.
A. Find the equation of the line.
B. What is the molar absorptivity of Cl2 (FW = 70.9) at 250 nm?
C. Calculate the free chlorine residual of the sample as ppm Cl2.
D. Calculate ppm BaCl2 (FW = 208.2)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning