The concentration is 0.045 molar and the average volume is 0.358 What is the concentration of absorbic acid in the vitamin c solution of tablet 1? Round to 3 sig fig
The concentration is 0.045 molar and the average volume is 0.358 What is the concentration of absorbic acid in the vitamin c solution of tablet 1? Round to 3 sig fig
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 26P
Related questions
Question
The concentration is 0.045 molar and the average volume is 0.358
What is the concentration of absorbic acid in the vitamin c solution of tablet 1? Round to 3 sig fig
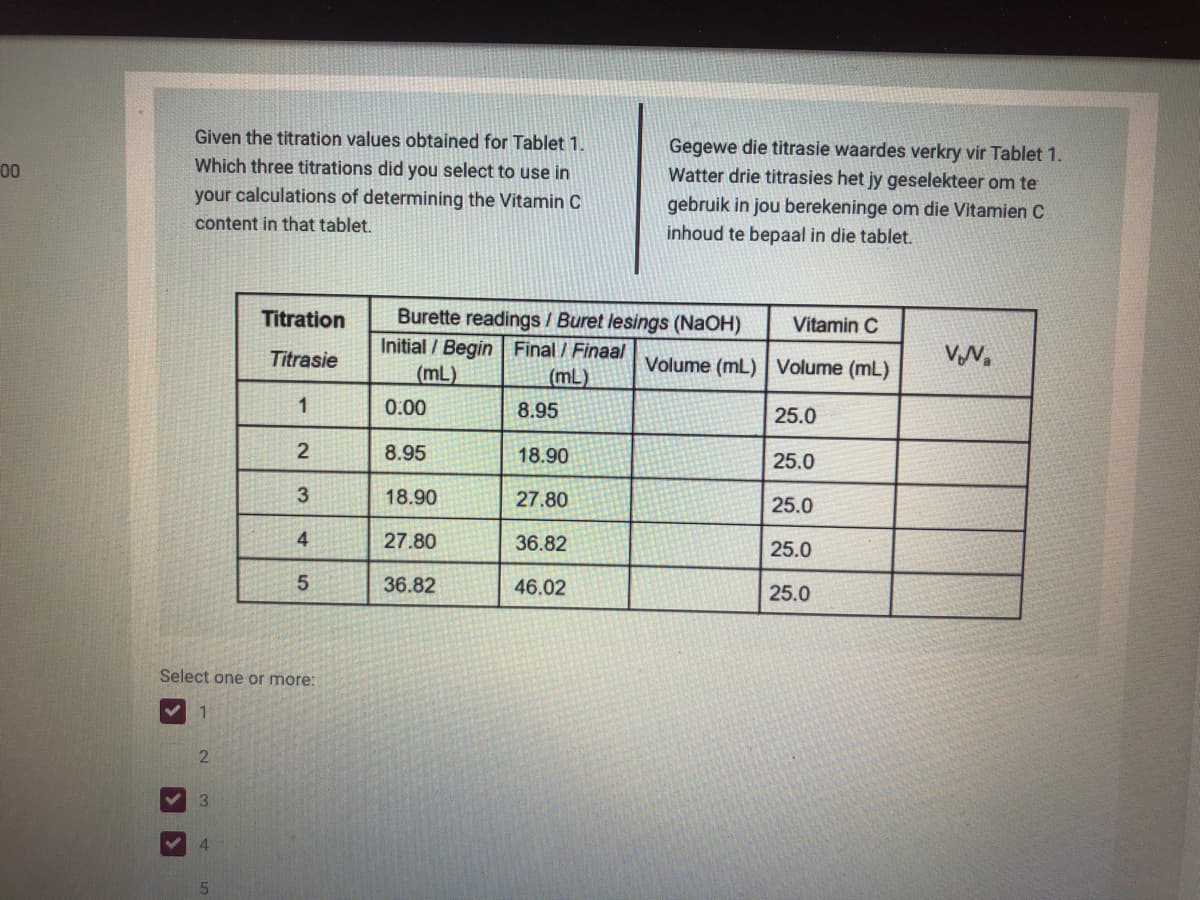
Transcribed Image Text:Given the titration values obtained for Tablet 1.
Gegewe die titrasie waardes verkry vir Tablet 1.
Watter drie titrasies het jy geselekteer om te
gebruik in jou berekeninge om die Vitamien C
inhoud te bepaal in die tablet.
00
Which three titrations did you select to use in
your calculations of determining the Vitamin C
content in that tablet.
Burette readings / Buret lesings (NaOH)
Initial / Begin Final / Finaal
(mL)
Titration
Vitamin C
Titrasie
Volume (mL) | Volume (mL)
VN.
(mL)
1
0.00
8.95
25.0
2
8.95
18.90
25.0
3
18.90
27.80
25.0
4
27.80
36.82
25.0
36.82
46.02
25.0
Select one or more:
4.
5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning