The drive-side airbag deploys when a car is in a head-on collision. The airbag inflates to a volume of 66.7 L and a pressure of 1.66 bar at 25.0 °C. Calculate the mass of NaN3, in grams, that the manufacturer placed into the gas generator to produce this amount of nitrogen gas. Assume the nitrogen gas behaves as an ideal gas and the only source of the nitrogen gas is reaction 1. Refer to the list of physical constants.
The drive-side airbag deploys when a car is in a head-on collision. The airbag inflates to a volume of 66.7 L and a pressure of 1.66 bar at 25.0 °C. Calculate the mass of NaN3, in grams, that the manufacturer placed into the gas generator to produce this amount of nitrogen gas. Assume the nitrogen gas behaves as an ideal gas and the only source of the nitrogen gas is reaction 1. Refer to the list of physical constants.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section10.2: U. S/ Energy Sources And Consumption
Problem 10.4E
Related questions
Question

Transcribed Image Text:The drive-side airbag deploys when a car is in a head-on collision. The airbag inflates to a volume of 66.7 L and a pressure
of 1.66 bar at 25.0 °C. Calculate the mass of NaN3, in grams, that the manufacturer placed into the gas generator to
produce this amount of nitrogen gas. Assume the nitrogen gas behaves as an ideal gas and the only source of the nitrogen
gas is reaction 1. Refer to the list of physical constants.
mass of NaN3:
g
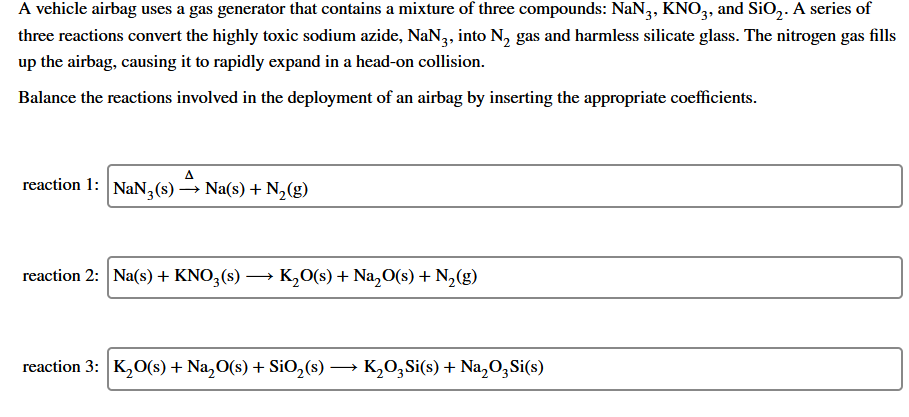
Transcribed Image Text:A vehicle airbag uses a gas generator that contains a mixture of three compounds: NaN,, KNO,, and SiO,. A series of
three reactions convert the highly toxic sodium azide, NaN3, into N, gas and harmless silicate glass. The nitrogen gas fills
up the airbag, causing it to rapidly expand in a head-on collision.
Balance the reactions involved in the deployment of an airbag by inserting the appropriate coefficients.
reaction 1: NaN3(s)
→ Na(s) + N,(g)
reaction 2: Na(s) + KNO,(s) → K,0(s) + Na,O(s) + N2(g)
reaction 3: K,O(s) + Na,O(s) + SiO,(s) –
K,0,Si(s) + Na,O,Si(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT