The following is intended for students who have already covered IR spectroscopy (Chapter 14). Consider the figure shown in Problem 7.91: Ph Ph ROH -Br Ph- -OR Ph Ph Ph 2 Ph = 3- 2a: R = H 2b: R= CH3 2c: R = CH2CH3 1 IR spectroscopy is an ideal tool for monitoring the conversion of 1 to 2a, while other forms of spectroscopy will be better suited for monitoring the conversion of 1 to 2b or 1 to 2c. Select the reasons why IR is an ideal tool ONLY for conversion of 1 to 2a. Because the O-H absorbance is in the fingerprint region, it is the best way to identify the product. O The CH3 group absorbs only in the fingerprint region so it is hard to identify. O An OH absorbance is diagnostically useful but C-Br and C-O absorbances are in the fingerprint region. C-Br and C-O absorbances are diagnostically useful but the O-H absorbance is in the fingerprint region.
The following is intended for students who have already covered IR spectroscopy (Chapter 14). Consider the figure shown in Problem 7.91: Ph Ph ROH -Br Ph- -OR Ph Ph Ph 2 Ph = 3- 2a: R = H 2b: R= CH3 2c: R = CH2CH3 1 IR spectroscopy is an ideal tool for monitoring the conversion of 1 to 2a, while other forms of spectroscopy will be better suited for monitoring the conversion of 1 to 2b or 1 to 2c. Select the reasons why IR is an ideal tool ONLY for conversion of 1 to 2a. Because the O-H absorbance is in the fingerprint region, it is the best way to identify the product. O The CH3 group absorbs only in the fingerprint region so it is hard to identify. O An OH absorbance is diagnostically useful but C-Br and C-O absorbances are in the fingerprint region. C-Br and C-O absorbances are diagnostically useful but the O-H absorbance is in the fingerprint region.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section20.3: Uv-visible Spectroscopy
Problem 20.5P
Related questions
Question
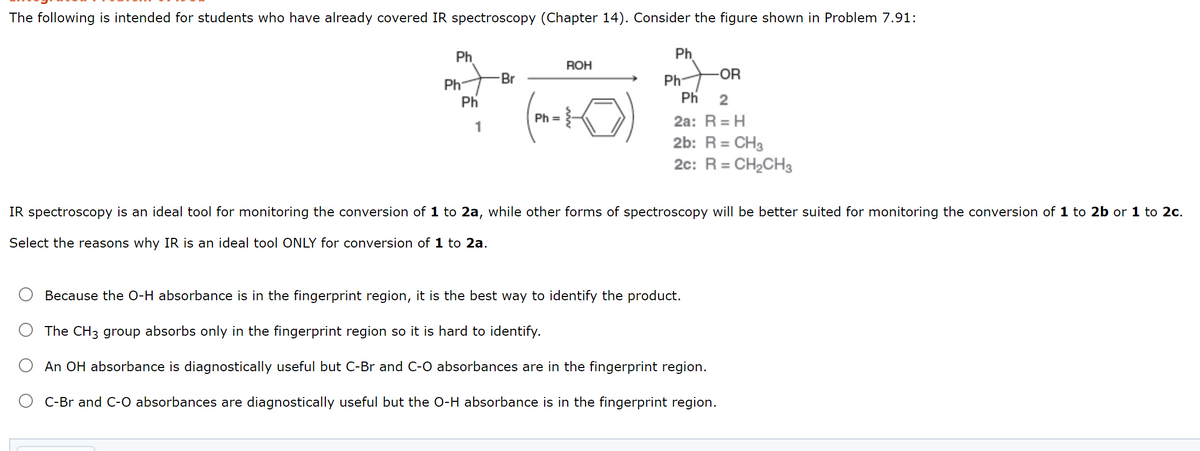
Transcribed Image Text:The following is intended for students who have already covered IR spectroscopy (Chapter 14). Consider the figure shown in Problem 7.91:
Ph
Ph
ROH
Br
Ph-
-OR
Ph
Ph
Ph
2
Ph =
2a: R = H
1
2b: R = CH3
2c: R = CH2CH3
IR spectroscopy is an ideal tool for monitoring the conversion of 1 to 2a, while other forms of spectroscopy will be better suited for monitoring the conversion of 1 to 2b or 1 to 2c.
Select the reasons why IR is an ideal tool ONLY for conversion of 1 to 2a.
Because the O-H absorbance is in the fingerprint region, it is the best way to identify the product.
The CH3 group absorbs only in the fingerprint region so it is hard to identify.
An OH absorbance is diagnostically useful but C-Br and C-O absorbances are in the fingerprint region.
C-Br and C-O absorbances are diagnostically useful but the O-H absorbance is in the fingerprint region.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning



Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning



Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning